Abstract
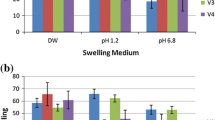
Blend microspheres of chitosan and polyurethane (PU) have been prepared by water-in-oil emulsion cross-linking method and used to encapsulate two water-soluble and having widely different plasma half-life cardiovascular drugs, viz., isoxsuprine hydrochloride and calcium dobesilate. The blend miscibility of the polymers was confirmed by differential scanning calorimetry at >60 wt% of PU. The microspheres were characterized by scanning electron microscopy to understand the morphology of the drug-loaded microspheres. Chemical interactions between drug molecules and the carrier polymers have been investigated by Fourier transform spectroscopy. XRD measurements on placebo matrices, drug-loaded formulations and nascent drugs indicated their uniform dispersion in the polymer matrix. In vitro release experiments performed in both acidic pH of 1.2 and alkaline pH of 7.4 increased the release time of both the drugs in the media employed. Kinetics of drug release was analyzed by empirical equation, suggesting the deviation from Fickian transport to non-Fickian trend.








Similar content being viewed by others
References
Liu Y, Messmer MC (2003) Structures and segregation of polystyrene/poly(methyl methacrylate) blends studied by sum-frequency (SF) spectroscopy. J Phys Chem B 107:9774
Zhang X, Kale DM, Jenekhe SA (2002) Electroluminescence of multicomponent conjugated polymers. 2. Photophysics and enhancement of electroluminescence from blends of polyquinolines. Macromolecules 35:382
Radhakrishnan J, Tanigaki N, Kaito A (1999) Electronic energy transfer in compatible blends of poly(di-n-hexylsilane) and poly(methyl-n-propylsilane). Polymer 40:1381
Kurkuri MD, Nayak JN, Aralaguppi MI, Naidu BVK, Aminabhavi TM (2005) Sorption/diffusion of aqueous mixtures of 1,4-dioxane/tetrahydrofuran through blend membranes of poly(vinyl alcohol) and sodium alginate: their compatibility and pervaporation separation studies. J Appl Polym Sci 98:178
Siepmann F, Siepmann J, Walther M, MacRae RJ, Bodmeier R (2008) Polymer blends for controlled release coatings. J Control Release 125:1
Karavas E, Georgarakis E, Bikiaris D (2006) Application of PVP/HPMC miscible blends with enhanced mucoadhesive properties for adjusting drug release in predictable pulsatile chronotherapeutics. Eur J Pharm Biopharm 64:115
Kerres J, Ullrich A, Meier F, Häring T (1999) Synthesis and characterization of novel acid–base polymer blends for application in membrane fuel cells. Solid State Ionics 125:243
Manea C, Mulder M (2002) Characterization of polymer blends of polyethersulfone/sulfonated polysulfone and polyethersulfone/sulfonated polyetheretherketone for direct methanol fuel cell applications. J Membr Sci 206:443
Kalyani S, Smitha B, Sridhar S, Krishnaiah A (2006) Blend membranes of sodium alginate and hydroxyethylcellulose for pervaporation-based enrichment of t-butyl alcohol. Carbohydr Polym 64:425
Bergera J, Reista M, Mayera JM, Feltb O, Peppasc NA, Gurny R (2004) Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. J Pharm Biopharm 57:19
Aminabhavi TM, Rudzinski WE (2010) Chitosan as a carrier for targeted delivery of small interfering RNA. Int J Pharm 399:1
Al-Qadi S, Grenha A, Carrión-Recio D, Seijo B, Remuñán-López C (2012) Microencapsulated chitosan nanoparticles for pulmonary protein delivery: in vivo evaluation of insulin-loaded formulations. J Control Release 157:383
Park JH, Saravanakumar G, Kim K, Kwon IC (2010) Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Del Rev 62:28
Kim TH, Jiang HL, Jere D, Park IK, Cho MH, Nah JW, Choi YJ, Akaike T, Cho CS (2007) Chemical modification of chitosan as a gene carrier in vitro and in vivo. Progr Polym Sci 32:726
Prabaharan M (2008) Chitosan derivatives as promising materials for controlled drug delivery. J Biomater Appl 23:5
Kidane AG, Burriesci G, Edirisinghe M, Ghanbari H, Bonhoeffer P, Seifalian AM (2009) A novel nanocomposite polymer for development of synthetic heart valve leaflets. Acta Biomater 5:2409
Xue L, Greisler HPJ (2003) Biomaterials in the development and future of vascular grafts. Vasc Surg 37:472
Rosenheck S, Sharon Z, Leibowitz D (2000) Artifacts recorded through failing bipolar polyurethane insulated permanent pacing leads. Europace 2:60
Lawrence EL, Turner IG (2005) Materials for urinary catheters: a review of their history and development in the UK. Med Eng Phys 27:443
Yang MJ, Den XY, Zhang Z, Julien M, Pelletier F, Desaulniers D, Cossette R, Teijeira FJ, Laroche G, Guidoin R (1997) Are intraaortic balloons suitable for reuse? A survey study of 112 used intraaortic balloons. Artif Organs 21:121
Ghosh B, Urban MW (2009) Self-repairing oxetane-substituted chitosan polyurethane networks. Science 323:1458
Gisselfalt K, Edberg B, Flodin P (2002) Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules 3:951
Zhang CH, Wen XJ, Vyavahare NR, Boland T (2008) Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials 29:3781
Adhikari R, Gunatillake PA, Griffiths I, Tatai L, Wickramaratna M, Houshyar S, Moore T, Mayadunne RTM, Field J, Mcgee M, Carbone T (2008) Biodegradable injectable polyurethanes: synthesis and evaluation for orthopaedic applications. Biomaterials 29:3762
Kang WM, Cheng BW, Li QX, Zuo FF (2010) Novel antibacterial nanofibers of chitosan and polyurethane prepared by electrospinning. Adv Mat Res 150–151:1452
Lijuan Z, Lunquan Y, Mingming D, Jiehua L, Hong T, Zhigao W, Qiang F (2011) Synthesis and characterization of pH-sensitive biodegradable polyurethane for potential drug delivery applications. Macromolecules 44:857
Okada M (2002) Chemical syntheses of biodegradable polymers. Prog Polym Sci 27:87
Zhang JN, Wu MY, Yang JJ, Wu QY, Jin ZL (2009) Anionic poly (lactic acid)-polyurethane micelles as potential biodegradable drug delivery carriers. Colloids Surf A 337:200
Shelke NB, Sairam M, Halligudi SB, Aminabhavi TM (2007) Development of transdermal drug-delivery films with castor-oil-based polyurethanes. J Appl Polym Sci 103:779
Shelke NB, Aminabhavi TM (2007) Synthesis and characterization of methoxypolyethyleneglycol and lauric acid grafted novel polyurethanes for controlled release of nifedipine. J Appl Polym Sci 105:2155
Costa-Júnior ES, Barbosa-Stancioli EF, Mansur AAP, Vasconcelos WL, Mansur HS (2009) Preparation and characterization of chitosan/poly(vinyl alcohol) chemically crosslinked blends for biomedical applications. Carbohydr Polym 76:472
Torre PMDL, Enobakhare, Torrado YG, Torrado S (2003) Release of amoxicillin from polyionic complexes of chitosan and poly(acrylic acid). Study of polymer/polymer and polymer/drug interactions within the network structure. Biomaterials 24:1499
Nafee N, Taetz S, Schneider M, Schaefer UF, Lehr CM (2007) Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomed Nanotechnol Biol Med 3:173
Alves NM, Mano JF (2008) Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int J Biolog Macromol 43:401
Jayakumar R, Prabaharan M, Nair SV, Tokura S, Tamura H, Selvamurugan N (2010) Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Progr Mater Sci 55:675
Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS (2008) Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 26:1
Kumbar SG, Kulkarni AR, Aminabhavi TM (2002) Crosslinked chitosan microspheres for encapsulation of diclofenac sodium: effect of crosslinking agent. J Microencapsul 19:173
Ramesh Babu V, Hosamani KM, Aminabhavi TM (2008) Preparation and in-vitro release of chlorothiazide novel pH-sensitive chitosan-N,N'-dimethylacrylamide semi-interpenetrating network microspheres. Carbohydr Polym 71:208
Reddy KM, Ramesh Babu V, Krishna Rao KSV, Subha MCS, Chowdoji Rao K, Sairam M, Aminabhavi TM (2008) Temperature sensitive semi-IPN microspheres from sodium alginate and N-isopropylacrylamide for controlled release of 5-fluorouracil. J Appl Polym Sci 107:2820–2829
Rokhade AP, Kulkarni PV, Mallikarjuna NN, Aminabhavi TM (2009) Preparation and characterization of novel semi-interpenetrating polymer network hydrogel microspheres of chitosan and hydroxypropyl cellulose for controlled release of chlorothiazide. J Microencapsul 26:27–36
Sullad AG, Manjeshwar LS, Aminabhavi TM (2010) Polymeric blend microspheres for controlled release of theophylline. J Appl Polym Sci 117:1361–1370
NTP (1999) Toxicology and carcinogenesis studies of glutaraldehyde (CAS No. 111-30-8) in F344/N rats and B6C3F1mice (inhalation studies). Natl Toxicol Progr Tech Rep Ser 490:1
Dhawan S, Singla AK, Sinha VR (2004) Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS Pharm Sci Tech 5:1–7
Kajjari PB, Manjeshwar LS, Aminabhavi TM (2011) Semi-interpenetrating polymer network hydrogel blend microspheres of gelatin and hydroxyethyl cellulose for controlled release of theophylline. Ind Eng Chem Res 50:7833–7840
Sullad AG, Manjeshwar LS, Aminabhavi TM (2010) Controlled release of theophylline from interpenetrating blend microspheres of poly(vinyl alcohol) and methyl cellulose. J Appl Polym Sci 116:1226–1235
Luo K, Yin J, Khutoryanskaya OV, Khutoryanskiy VV (2008) Mucoadhesive and elastic films based on blends of chitosan and hydroxyethylcellulose. Macromol Biosci 8:184–192
He P, Davis SS, Illum L (1999) Chitosan microspheres prepared by spray drying. Int J Pharm 187:53–65
Al-Helw AA, Al-Angary AA, Mahrous GM, Al-Dardari MM (1998) Preparation and evaluation of sustained release cross-linked chitosan microspheres containing phenobarbitone. J Microencapsul 15:373–382
Rokhade AP, Shelke NB, Patil SA, Aminabhavi TM (2007) Novel interpenetrating polymer network microspheres of chitosan and methylcellulose for controlled release of theophylline. Carbohydr Polym 69:678–687
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42
Sullad AG, Manjeshwar LS, Aminabhavi TM (2010) Novel pH-sensitive hydrogels prepared from the blends of poly(vinyl alcohol with acrylic acid-graft-guar gum matrixes for isoniazid delivery. Ind Eng Chem Res 49:7323–7329
Acknowledgments
Miss. A. G. Sullad and Prof. L. S. Manjeshwar thank the University Grants Commission (UGC), New Delhi, India (KU/SCH/UGC/RFSMS/2008-09). Prof. T. M. Aminabhavi thanks the All India Council for Technical Education [AICTE, F.No. 1-51/RIFD/EF(13)/2011-12], New Delhi, India for the award of Emeritus Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sullad, A.G., Manjeshwar, L.S. & Aminabhavi, T.M. Blend microspheres of chitosan and polyurethane for controlled release of water-soluble antihypertensitive drugs. Polym. Bull. 72, 265–280 (2015). https://doi.org/10.1007/s00289-014-1271-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1271-6