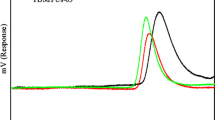
Abstract
Polyurethane (PU) grafted with several well-known pH indicators (alizarin yellow, bromocresol green, bromocresol purple, or thymol blue) via spacer is characterized for thermal, spectroscopic, mechanical, and shape memory properties, as well as for PU color change to aqueous solution with various pH values. The PU polymer frame is composed of 4,4′-methylenebis(phenylisocyanate) (MDI), which acts as a hard segment, poly(tetramethyleneglycol) (PTMG) as a soft segment, and a covalently linked pH indicator. The four different PU series studied in this work display characteristic color dependent upon the grafted indicator type. The PU series also exhibit a small degree of cross-linking due to the grafting agent used to covalently link the indicator to the PU frame. The tensile mechanical strength and the shape recovery of the indicator-grafted PU remain high after repeated tests compared to that of the plain linear PU. Regarding the tensile mechanical properties, the maximum stress and the strain increase to 424 and 1,880 %, respectively, for indicator-grafted PU compared to the linear PU. Furthermore, the shape recovery is observed to reach 98 % and improves after each test cycle. A reversible color change is observed after repeated exposure to aqueous solutions with varying pH values and is confirmed with UV-VIS spectra results.












Similar content being viewed by others
References
Bowles SE, Wu W, Kowalewski T, Schalnat MC, Davis RJ, Pemberton JE, Shim I, Korth BD, Pyun J (2007) Magnetic assembly and pyrolysis of functional ferromagnetic colloids into one dimensional carbon nanostructures. J Am Chem Soc 29:8694–8695
Yager KG, Barrett CJ (2006) Novel photo-switching using azobenzene functional materials. J Photochem Photobiol A 182:250–261
Filipcsei G, Fehér J, Zrinyi M (2000) Electric field sensitive neutral polymer gels. J Mol Struct 554:109–117
Mano JF (2008) Stimuli-responsive polymeric systems for biomedical applications. Adv Eng Mater 10:515–527
Ganta S, Devalapally H, Shahiwala A et al (2008) A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release 126:187–204
Roy D, Cambre JN, Sumerlin BS (2009) Triply-responsive boronic acid block copolymers: solution self-assembly induced by changes in temperature, pH, or sugar concentration. Chem Commun 16:2106–2108
Chung YC, Park HS, Choi JW, Chun BC (2012) Characterization and low temperature test of the flexibly crosslinked polyurethane copolymer by poly(dimethylsiloxane). High Perform Polym 24:200–209
Chung YC, Park JS, Shin CH, Choi JW, Chun BC (2012) Grafting of shape memory polyurethane with poly(ethyleneimine) and the effect on electrolytic attraction in aqueous solution and shape recovery properties. Macromol Res 20:66–75
Chung YC, Choi JW, Choi MW, Chun BC (2012) Characterization of flexibly linked shape memory polyurethane composite with magnetic property. J Thermoplast Compos 25:283–303
Chung YC, Cho TK, Chun BC (2009) Flexible cross-linking by both pentaerythritol and polyethyleneglycol spacer and its impact on the mechanical properties and the shape memory effects of polyurethane. J Appl Polym Sci 112:2800–2808
Chung YC, Choi JW, Lee SH, Chun BC (2011) Investigation of fluorescent shape memory polyurethane grafted with various dyes. Bull Korean Chem Soc 32:2988–2996
Takahashi T, Hayashi N, Hayashi S (1996) Structure and properties of shape-memory polyurethane block copolymers. J Appl Polym Sci 60:1061–1069
Chen LW, Lin JR (1998) Study on shape-memory behavior of polyether-based polyurethanes. II. Influence of soft-segment molecular weight. J Appl Polym Sci 69:1575–1586
Wang W, Ping P, Chen X, Jing X (2006) Polylactide-based polyurethane and its shape memory behavior. Eur Polym J 42:1240–1249
Christie RM, Bryant ID (2005) An evaluation of thermochromic prints based on microencapsulated liquid crystals using variable temperature colour measurement. Color Technol 121:187–192
Reduwan SM, Billah SM, Christiea RM, Shamey R (2008) Direct coloration of textiles with photochromic dyes. Part 1: application of spiroindolinonaphthoxazines as disperse dyes to polyester, nylon and acrylic fabrics. Color Technol 124:223–228
Van der Schueren L, De Clerck K (2010) The use of pH-indicator dyes for pH sensitive textile materials. Text Res J 80:590–603
Staneva D, Betcheva R, Chovelon JM (2007) Optical sensor for aliphatic amines based on the simultaneous colorimetric and fluorescence responses of smart textile. J Appl Polym Sci 106:1950–1956
Van der Schueren L, Hemelsoet K, Van Speybroeck V, De Clerck K (2012) The influence of a polyamide matrix on the halochromic behaviour of the pH-sensitive azo dye Nitrazine Yellow. Dyes Pigm 94:443–451
Staneva D, Betcheva R (2007) Synthesis and functional properties of new optical pH sensor based on benzo[de]anthracen-7-one immobilized on the viscose. Dyes Pigm 74:148–153
Denizlia A, Say R, Piskin E (2003) Removal of aluminium by Alizarin Yellow-attached magnetic poly(2-hydroxyethyl methacrylate) beads. React Funct Polym 55:99–107
Jurmanović S, Kordić Š, Steinberg MD, Steinberg IM (2010) Organically modified silicate thin films doped with colourimetric pH indicators methyl red and bromocresol green as pH responsive sol–gel hybrid materials. Thin Solid Films 518:2234–2240
Ibarra JC, Ortiz-Gutie′rrez M, Alonso-Magana P (2004) Characterization of bromocresol green and resin as holographic film. Opt Mater 27:567–572
Van der Schueren L, Mollet T, Ceylan Ö, De Clerck K (2010) The development of polyamide 6.6 nanofibres with a pH-sensitive function by electrospinning. Eur Polym J 46:2229–2239
Wang Y, Tong L–L (2010) Electrochemical sensor for simultaneous determination of uric acid, xanthine and hypoxanthine based on poly (bromocresol purple) modified glassy carbon electrode. Sens Actuators B Chem 150:43–49
Miled OB, Ouada HB, Livage J (2002) pH sensor based on a detection sol gel layer onto optical fiber. Mater Sci Eng C 21:183–188
Kitajyo Y, Imai T, Sakai Y, Tamaki M, Tani H, Takahashi K, Narumi A, Kaga H, Kaneko N, Satoh T, Kakuchi T (2007) Encapsulation-release property of amphiphilic hyperbranched D glucan as a unimolecular reverse micelle. Polymer 48:1237–1244
Freij-Larsson C, Wesslen B (1993) Grafting of polyurethane surfaces with poly(ethyleneglycol). J Appl Polym Sci 50:345–352
Archambault JG, John L (2004) Protein resistant polyurethane surfaces by chemical grafting of PEO: amino-terminated PEO as grafting reagent. Colloid Surf B 39:9–16
Tan K, Obendorf SK (2006) Surface modification of microporous polyurethane membrane with poly(ethylene glycol) to develop a novel membrane. J Membr Sci 274:150–158
Alves P, Coelho JFJ, Haack J, Rota A, Bruinink A, Gil MH (2009) Surface modification and characterization of thermoplastic polyurethane. Eur Polym J 45:1412–1419
Huang J, Xu W (2010) Zwitterionic monomer graft copolymerization onto polyurethane surface through a PEG spacer. Appl Surf Sci 256:3921–3927
Petrovic ZS, Javni I, Divjakovic VJ (1998) Structure and physical properties of segmented polyurethane elastomers containing chemical crosslinks in the hard segment. J Polym Sci Polym Phys 36:221–235
Sekkar V, Gopalakrishnan S, Devi KA (2003) Studies on allophanate–urethane networks based on hydroxyl terminated polybutadiene: effect of isocyanate type on the network characteristics. Eur Polym J 39:1281–1290
Sekkar V, Rama Rao M, Krishinamurthy VN, Jane SR (1996) Modeling of polyurethane networks based on hydroxy-terminated polybutadiene and poly(12-hydroxy stearic acid–co TMP) ester polyol: correlation of network parameters with mechanical properties. J Appl Polym Sci 62:2317–2327
Chung YC, Choi JW, Chung HM, Chun BC (2012) The MDI-mediated lateral crosslinking of polyurethane copolymer and the impact on tensile properties and shape memory effect. Bull Korean Chem Soc 33:692–694
Hu J, Yang Z, Yeung L, Ji F, Liu Y (2005) Crosslinked polyurethanes with shape memory properties. Polym Int 54:854–859
Zhuohonga Y, Jinliana H, Yeqiua L, Lapyana Y (2006) The study of crosslinked shape memory polyurethanes. Mater Chem Phys 98:368–372
Silva A, Boto REF, El-Shishtawy RM, Almeida P (2006) Rhodamine B as ligand for affinity chromatography. Fixation studies onto cellulose by a curing method. Eur Polym J 42:2270–2282
Cerruti P, Borbone F, Carella A, Malinconico M, Mormile P, Petti L, Rippa M, Roviello A, Laurienzo P (2009) Covalent attachment of chromophores to chlorinated copolymers for optical waveguides: synthesis and optical characterization. Polymer 50:1645–1653
Carreón-Castro MP, Rivera E, Cruz JJ, Zavaleta G, Gutiérrez-Nava M (2010) Preparation and characterization of grafted polyethylene based azo-polymer films. Thin Solid Films 518:4136–4141
Acknowledgments
This study was supported by the R&D Center for Valuable Recycling (Global-Top Environmental Technology Development Program) funded by the Ministry of Environment (Project No.: 11-E29-IR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, YC., Jung, IH., Choi, J.W. et al. Characterization and proof testing of the halochromic shape memory polyurethane. Polym. Bull. 71, 1153–1171 (2014). https://doi.org/10.1007/s00289-014-1116-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1116-3