Abstract
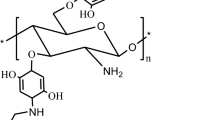
A novel derivative of chitosan (CTS), ortho-biguanidinyl benzoyl chitosan hydrochloride (o-BGBCH), was prepared with CTS and ortho-biguanidinyl benzoyl chloride, which was synthesized by acid chloride reaction of ortho-biguanidinyl benzonic acid hydrochloride (o-BGBA), as starting material in the medium consisted of methylsulfonic acid (MeSO3H) and dimethyl sulfoxide. Structure of o-BGBCH was characterized by FT-IR and 1H NMR, molecular weight of o-BGBCH was determined through gel permeation chromatography, and degree of substitution (DS) of guanidinylation of o-BGBCH was measured using elemental analysis technique. Meanwhile, the minimum inhibitory concentration of o-BGBCHs against Escherichia coli, a Gram-negative bacterium, and Staphylococcus aureus, a Gram-positive bacterium, were ascertained by agar plate method. Compared with CTS hydrochloride, o-BGBCHs had much stronger antimicrobial activities, and these activities improved with the increase of their DS of guanidinylation. When the DS of o-BGBCH reached or exceeded 32.7 %, its antibacterial activities against the tested bacteria were higher than that of Bromo-Geramium.








Similar content being viewed by others
References
Chen CY, Lin HC, Huang YY, Chen KL, Huang JJ, Yeh MY, Wong FF (2010) ‘One-flask’ transformation of isocyanates and isothiocyanates to guanidines hydrochloride by using sodium bis(trimethylsilyl)amide. Tetrahedron 66:1892–1897
Shinada T, Umezawa T, Ando T, Kozuma H, Ohfune Y (2006) A new entry for the synthesis of N-acyl-N′-substituted guanidines. Tetrahedron Lett 47:1945–1947
Schroif-Grégoire C, Barale K, Zaparucha A, Al-Mourabit A (2007) Preparation of N-alkyl-N′-carboalkoxy guanidines: unexpected effective trans-alkoxylation transforming the 2,2,2-trichloroethoxycarbonyl into various carbamates. Tetrahedron Lett 48:2357–2359
Arafa RK, Ismail MA, Munde M, Wilson WD, Wenzler T, Brun R, Boykin DW (2008) Novel linear triaryl guanidines, N-substituted guanidines and potential prodrugs as antiprotozoal agents. Eur J Med Chem 43:2901–2908
Dykens JA, Jamieson J, Marroquin L, Nadanaciva S, Billis PA, Will Y (2008) Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicol Appl Pharmacol 233:203–210
Lebrini M, Bentiss F, Chihib N-E, Jama C, Hornez JP, Lagrenée M (2008) Polyphosphate derivatives of guanidine and urea copolymer: inhibiting corrosion effect of armco iron in acid solution and antibacterial activity. Corros Sci 50:2914–2918
Nimesh S, Chandra R (2008) Guanidinium-grafted polyethylenimine: an efficient transfecting agent for mammalian cells. Eur J Pharm Biopharm 68:647–655
Matulková I, Nĕmec I, Císařová I, Nĕmec P, Mička Z (2008) Inorganic salts of biguanide—searching for new materials for second harmonic generation. J Mol Struct 886:103–120
Wang Y, Sauer DR, Djuric SW (2009) A facile and practical one-pot ‘catch and release’ synthesis of substituted guanidines. Tetrahedron Lett 50:5145–5148
Pi CF, Zhang ZX, Pang Z, Zhang J, Luo J, Chen ZX, Weng LH, Zhou XG (2007) Multiple NH bond activation: synthesis and reactivity of functionalized primary-multiple N amido ytterbium complexes. Organometallics 26:1934–1946
Jones C, Junk PC, Platts JA, Stasch A (2006) Four-membered group 13 Metal(I) N-heterocyclic carbene analogues: synthesis, characterization, and theoretical studies. J Am Chem Soc 128:2206–2207
Ube H, Uraguchi D, Terada M (2007) Efficient synthetic protocol for substituted guanidines via copper(I)-mediated intermolecular amination of isothiourea derivatives. J Organomet Chem 692:545–549
Qian L, Guan Y, He B, Xiao H (2008) Modified guanidine polymers: synthesis and antimicrobial mechanism revealed by AFM. Polymer 49:2471–2475
Zhang L-Y, Yao S-J, Guan Y-X (2005) Effects of poly(methylene-co-guanidine) on microbial growth in an alginate/cellulose sulphate–CaCl2/poly(methylene-co-guanidine) capsule system. Process Biochem 40:189–193
Sun S, An Q, Li X, Qian L, He B, Xiao H (2010) Synergistic effects of chitosan–guanidine complexes on enhancing antimicrobial activity and wet-strength of paper. Bioresour Technol 101:5693–5700
Hu Y, Du Y, Yang J, Kennedy JF, Wang X, Wang L (2007) Synthesis, characterization and antibacterial activity of guanidinylated chitosan. Carbohydr Polym 67:66–72
Tang H, Zhang P, Kieft TL, Ryan SJ, Baker SM, Wiesmann WP, Rogelj S (2010) Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta Biomater 6:2562–2571
Sashiwa H, Thompson JM, Das SK, Shigemasa Y, Tripathy S, Roy R (2000) Chemical modification of chitosan: preparation and lectin binding properties of r-galactosyl-chitosan conjugates. Potential inhibitors in acute rejection following xenotransplantation. Biomacromolecules 1:303–305
Yue W, Yao P, Wei Y (2009) Influence of ultraviolet-irradiated oxygen on depolymerization of chitosan. Polym Degrad Stab 94:851–858
Ashmore M, Hearn J, Karpowicz F (2001) Flocculation of latex particles of varying surface charge densities by chitosans. Langmuir 17:1069–1073
Casal E, Corzo N, Moreno FJ, Olano A (2005) Selective recovery of glycosylated caseinmacropeptide with chitosan. J Agric Food Chem 53:1201–1204
Park S-I, Zhao Y-Y (2004) Incorporation of a high concentration of mineral or vitamin into chitosan-based films. J Agric Food Chem 52:1933–1939
Jeon Y-J, Kamil JYVA, Shahidi F (2002) Chitosan as an edible invisible film for quality preservation of herring and atlantic cod. J Agric Food Chem 50:5167–5178
Sun L, Du Y, Fan L, Chen X, Yang J (2006) Preparation, characterization and antimicrobial activity of quaternized carboxymethyl chitosan and application as pulp-cap. Polymer 47:1796–1804
Hsieh C-Y, Hsieh H-J, Liu H-C, Wang D-M, Hou L-T (2006) Fabrication and release behavior of a novel freeze-gelled chitosan/g-PGA scaffold as a carrier for rhBMP-2. Dent Mater 22:622–629
Hu S-G, Jou C-H, Yang M-C (2004) Biocompatibility and antibacterial activity of chitosan and collagen immobilized poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid). Carbohydr Polym 58:173–179
Hsieh C-Y, Tsai S-P, Wang D-M, Chang Y-N (2005) Preparation of g-PGA/chitosan composite tissue engineering matrices. Biomaterials 26:5617–5623
Tapia C, Corbalán V, Costa E, Gai MN, Yazdani-Pedram M (2005) Study of the release mechanism of diltiazem hydrochloride from matrices based on chitosan–alginate and chitosan–carrageenan mixtures. Biomacromolecules 6:2389–2395
Batista MKS, Pinto LF, Gomes CAR, Gomes P (2006) Novel highly-soluble peptide–chitosan polymers:chemical synthesis and spectral characterization. Carbohydr Polym 64:299–305
Sun L, Du Y, Yang J, Shi X, Li J, Wang X, Kennedy JF (2006) Conversion of crystal structure of the chitin to facilitate preparation of a 6-carboxychitin with moisture absorption–retention abilities. Carbohydr Polym 66:168–175
Rúnarsson ÖV, Holappa J, Malainer C, Steinsson H, Hjálmarsdóttir M, Nevalainen T, Másson M (2010) Antibacterial activity of N-quaternary chitosan derivatives: synthesis, characterization and structure activity relationship (SAR) investigations. Eur Polym J 46:1251–1267
Li Z, Zhuang XP, Liu XF, Guan YL, Yao KD (2002) Study on antibacterial O-carboxymethylated chitosan/cellulose blend film from N,N-dimethylacetamide solution. Polymer 43:1541–1547
Kogan G, Skorik YA, Žitňanová I, Križková L, Ďuračková Z, Gomes CAR, Yatlukf YG, Krajčovic J (2004) Antioxidant and antimutagenic activity of N-(2-carboxyethyl)chitosan. Toxicol Appl Pharmacol 201:303–310
Sajomsang W, Tantayanon S, Tangpasuthadol V, Daly WH (2009) Quaternization of N-aryl chitosan derivatives: synthesis, characterization, and antibacterial activity. Carbohydr Res 344:2502–2511
Xu Y, Du Y, Huang R, Gao L (2003) Preparation and modification of N-(2-hydroxyl)propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier. Biomaterials 24:5015–5022
Schatz C, Bionaz A, Lucas J-M, Pichot C, Viton C, Domard A, Delair T (2005) Formation of polyelectrolyte complex particles from self-complexation of N-sulfated chitosan. Biomacromolecules 6:1642–1647
Holappa J, Nevalainen T, Savolainen J, Soininen P, Elomaa M, Safin R, Suvanto S, Pakkanen T, Másson M, Loftsson T, Järvinen T (2004) Synthesis and characterization of chitosan N-betainates having various degrees of substitution. Macromolecules 37:2784–2789
Ma X, Li Y, Ye Z, Yang L, Zhou L, Wang L (2011) Novel chelating resin with cyanoguanidine group: useful recyclable materials for Hg(II) removal in aqueous environment. J Hazard Mater 185:1348–1354
Xie W, Xu P, Wang W, Liu Q (2002) Preparation and antibacterial activity of a water-soluble chitosan derivative. Carbohydr Polym 50:35–40
Ikeda T, Hirayama HH, Yamaguchi S, Tazuke P (1986) Polycationic biocides with pendant active group: molecular weight dependence of antibacterial activity. Antimicrob Agents Chemother 30:132–136
Acknowledgments
We gratefully acknowledged the support of National Natural Science Foundation of P.R. China (No. 31170543), 973 Program in Natural Basic Research Program of P.R. China (No.2007CB936300) and Jiangsu Natural Science Foundation (BK 2009293). We also gratefully acknowledged the support of China Pharmaceutical University and Changzou University in analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, Zs., Sun, Ym., Zhu, Xm. et al. Preparation and characterization of ortho-biguanidinyl benzoyl chitosan hydrochloride and its antibacterial activities. Polym. Bull. 70, 1085–1096 (2013). https://doi.org/10.1007/s00289-012-0883-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-012-0883-y