Abstract
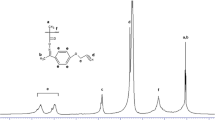
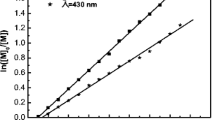
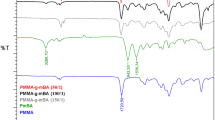
Novel optically active methacrylates polymer with controlled molecular weight have been prepared simply by opening ring reaction of poly(glycidyl methacrylate) and S-(−)-α-methylbenzylamine. The structure of the novel optically active methacrylates polymer was characterized by FT-IR, and chiroptical properties of the polymer were characterized by polarimeter and circular dichroism spectrum. Thermogravimetry analysis and differential scanning calorimetry were employed to examine the thermal properties of the polymer. This preparation process was simple and the racemization of optically active monomers in preparation can be avoided. The molecular weight of optically active methacrylates polymer could be controlled by molecular weight of poly(glycidyl methacrylate) prepared by atom transfer radical polymerization.







Similar content being viewed by others
References
Gohsh S, Krishnamurti N (2000) Use of glycidyl methacrylate monomers for developing crosslinkable pressure sensitive adhesives. Eur Polym J 36:2125–2131
Coessens V, Pintauer Y, Matyjaszewski K (2001) Functional polymer by atom transfer radical polymerization. Prog Polym Sci 26:337–377
Coessens V, Pyun J, Miller PJ, Gaynor SG, Matyjaszewski K (2000) Functionalization of polymers prepared by ATRP using radical addition reactions. Macromol Rapid Commun 21:103–109
Li G, Zhu X, Zhu J, Cheng Z, Zhang W (2005) Homogeneous reverse atom transfer radical polymerization of glycidyl methacrylate and ring-opening reaction of the pendant oxirane ring. Polymer 46:12716–12721
Loyens W, Groeninckx G (2003) Rubber toughened semicrystalline PET: influence of the matrix properties and test temperature. Polymer 44:123–136
Sanda F, Nakamura M, Endo T (1994) Synthesis of novel optically active polymethacrylamide having l-leucine structure in the side chain. Macromolecules 27:7928–7929
Nakano T (2001) Optically active synthetic polymers as chiral stationary phases in HPLC. J Chromatogr A 906:205–225
Angiolini L, Caretti D, Giorgini L, Salatelli E, Altomare A, Carlini C, Solaro R (2000) Optically active polymethacrylates with side-chain l-lactic acid residues connected to push–pull azobenzene chromophores. Polymer 41:4767–4780
Angiolini L, Caretti D, Giorgini L, Salatelli E (2000) Optically active methacrylic polymers bearing side-chain conjugated azoaromatic chromophores. Synth Met 115:235–239
Bush SM, North M (1996) Synthesis of homochiral addition polymers derived from N-trityl-(S)-serine. Polymer 37:4649–4752
Angiolini L, Giorgini L, Li H, Golemme A, Mauriello F, Termine R (2010) Synthesis, characterization and photoconductive properties of optically active methacrylic polymers bearing side-chain 9-phenylcarbazole moieties. Polymer 51:368–377
Angiolini L, Caretti D, Giorgini L, Salatelli E (2001) Methacrylic polymers bearing side-chain permanent dipole azobenzene chromophores spaced from the main chain by chiral moieties: synthesis and characterization. Polymer 42:4005–4016
Mallakpour S, Habibi S (2003) Microwave-promoted synthesis of new optically active poly(ester-imide)s derived from N, N′-(pyromellitoyl)-bis-l-leucine diacid chloride and aromatic diols. Eur Polym J 39:1823–1829
Mallakpour S, Sepehri S (2008) Polycondensation of new optically active diacid with diisocyanates in the presence of tetrabutylammonium bromide as a green media under microwave heating. React Funct Polym 68:1459–1466
Mallakpour S, Kolahdoozan M (2007) Synthesis and properties of thermally stable and optically active novel wholly aromatic polyesters containing a chiral pendent group. Eur Polym J 43:3344–3354
Krishnan R, Srinivasan KSV (2003) Controlled/“living” radical polymerization of glycidyl methacrylate at ambient temperature. Macromolecules 36:1769–1771
Krishnan R, Srinivasan KSV (2004) Room temperature atom transfer radical polymerization of glycidyl methacrylate mediated by copper(I)/N-alkyl-2-pyridylmethanimine complexes. Macromolecules 37:3614–3622
Haddleton DM, Jasieczek CB, Hannon MJ, Shooter AJ (1997) Atom transfer radical polymerization of methyl methacrylate initiated by alkyl bromide and 2-pyridinecarbaldehyde imine copper(I) complexes. Macromolecules 30:2190–2193
Haddleton DM, Kukulj D, Duncalf DJ, Heming AM, Shooter AJ (1998) Low-temperature living “radical” polymerization (atom transfer polymerization) of methyl methacrylate mediated by copper(I) N-alkyl-2-pyridylmethanimine complexes. Macromolecules 31:5201–5205
Çaykaraa T, Alaslana ŞŞ, Gürü M, Bodugöz H, Güven O (2007) Preparation and characterization of poly(isobutyl methacrylate) microbeads with grafted amidoxime groups. Radiat Phys chem 76:1569–1576
Çelik SÜ, Bozkurt A (2008) Preparation and proton conductivity of acid-doped 5-aminotetrazole functional poly(glycidyl methacrylate). Eur Polym J 44:213–218
Nanjundan S, Unnithan CS, Selvamalar CSJ, Penlidis A (2005) Homopolymer of 4-benzoylphenyl methacrylate and its copolymers with glycidyl methacrylate: synthesis, characterization, monomer reactivity ratios and application as adhesives. React Funct Polym 62:11–24
Aslan A, Çelik SÜ, Bozkurt A (2009) Proton-conducting properties of the membranes based on poly(vinyl phosphonic acid) grafted poly(glycidyl methacrylate). Solid State Ionics 180:1240–1245
Acknowledgment
We would like to gratitude for the financial support from the Central Universities Foundation of Harbin Engineering University, China (HEUCFT1009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X.J., Zhao, M. & Gu, S.S. Simple process for preparation of optically active methacrylates polymer with controlled molecular weight. Polym. Bull. 68, 1525–1535 (2012). https://doi.org/10.1007/s00289-011-0624-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-011-0624-7