Abstract
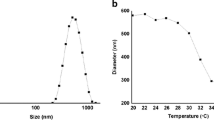
The pH-responsive swelling and release behaviors of anionic P(MAA-co-EGMA) hydrogel microparticles having various MAA and EG contents were investigated as a biological on–off switch for the design of an intelligent drug delivery system triggered by external pH changes. When DC was used as a dispersion stabilizer, well-dispersed hydrogel microparticles having an average diameter of approximately 4 μm were obtained. There was a drastic change of the equilibrium weight swelling ratio of P(MAA-co-EGMA) hydrogels at a pH of around 5, which is the pK a of PMAA. When the MAA content in the hydrogel increased, the swelling ratio increased at a pH above 5 due to the more electrostatic repulsion between the charged groups of MAA. The P(MAA-co-EGMA) hydrogel microparticles showed a pH-responsive release behavior. At low pH (pH 4.0) small amounts of Rh-B were released while at high pH (pH 6.0) relatively large amounts of Rh-B were released from the hydrogels. The difference in the released amount of Rh-B from the hydrogels between pH 4.0 and 6.0 decreased when the MAA content in the hydrogels decreased, which means that the pH-responsive release behavior of the P(MAA-co-EGMA) hydrogel microparticles is closely related to the pH-responsive swelling property of the hydrogel.






Similar content being viewed by others
References
Peppas NA, Bures P, Leobandung W, Ichikawa H (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 50:27–46
Bell CL, Peppas NA (1996) Water, solute and protein diffusion in physiologically responsive hydrogels of poly (methacrylic acid-g-ethylene glycol). J Control Release 39:1203–1218
Soppirnath KS, Aminabhavi TM (2002) Water transport and drug release study from cross-linked polyacrylamide grafted guar hydrogel microspheres for the controlled release application. Eur J Pharm Biopharm 53:87–98
Tada D, Tanabe T, Tachobana A, Yamauchi K (2005) Drug release from hydrogel containing albumin as crosslinker. J Biosci Bioeng 100:551–555
Scott RA, Peppas NA (1999) Highly crosslinked PEG-containing copolymers for sustained solute delivery. Biomaterials 20:1371–1380
Kim C, Lim S (2002) Recent progress in drug delivery systems for anticancer agents. Arch Pharm Res 25:229–239
Kim B, Flamme KL, Peppas NA (2003) Dynamic swelling behavior of pH-sensitive anionic hydrogels used for protein delivery. J Appl Polym Sci 89:1606–1613
Peppas NA (2004) Devices based on intelligent biopolymers for oral protein delivery. Int J Pharm 277:11–17
Kim B, Peppas NA (2003) In vitro release behavior and stability of insulin in complexation hydrogels as oral drug delivery carriers. Int J Pharm 266:29–37
Morishita M, Goto T, Peppas NA, Joseph JI, Torjman MC, Munsick C, Nakamura K, Yamagata T, Takayama K, Lowman AM (2004) Mucosal insulin delivery systems based on complexation polymer hydrogels: effect of particle size on insulin enteral absorption. J Control Release 97:115–124
Foss AC, Goto T, Morishita M, Peppas NA (2004) Development of acrylic-based copolymers for oral insulin delivery. Eur J Pharm Biopharm 57:163–169
Kim KC, Lee SJ (1989) pH-dependent swelling properties of methacrylic acid copolymer hydrogels. J Pharm Soc Kor 33:372–376
Donini C, Robinson DN, Colombo P, Giordano F, Peppas NA (2002) Preparation of poly(methacrylic acid-g-poly(ethylene glycol)) nanospheres from methacrylic monomers for pharmaceutical applications. Int J Pharm 245:83–91
He H, Li L, Lee LJ (2006) Photopolymerization and structure formation of methacrylic acid based hydrogels in water/ethanol mixture. Polymer 47:1612–1619
Kim B, Peppas NA (2003) Analysis of molecular interactions in poly(methacrylic acid-g-ethylene glycol) hydrogels. Polymer 44:3701–3707
Robinson DN, Peppas NA (2002) Preparation and characterization of pH-responsive poly(methacrylic acid-g-ethylene glycol) nanospheres. Macromolecules 35:3668–3674
Mchedlov-Petrossyan NO, Vodolazkaya NA, Doroshenko AO (2003) Ionic equilibria of fluorophores in organized solutions: the influence of micellar microenvironment on protolytic and photophysical properties of rhodamine B. J Fluoresc 13:235–248
Acknowledgments
This work was supported by 2010 Hongik University Research Fund and the Grant of Small and Medium Business Administration, Republic of Korea (No. S1065960).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, E., Kim, B. Preparation and characterization of pH-sensitive hydrogel microparticles as a biological on–off switch. Polym. Bull. 67, 67–76 (2011). https://doi.org/10.1007/s00289-010-0403-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-010-0403-x