Abstract
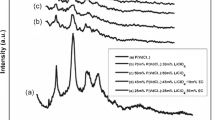
In this study, the water-soluble polymers of N-maleoyl glycine (MG) with crotonic acid (CA) were copolymerized by free radical polymerization to obtain hydrophilic polymers, in order to study the effect of the functional groups in the copolymers on the metal ion retention capacity, electrochemical and thermal behavior, since that important requirements for their use in technological applications are: high solubility in water, chemical stability, a high affinity for one or more metal ions, and selectivity for the metal ion of interest. The metal complexation properties of poly(MG-co-CA) for the metal ions were investigated at pH 3, 5, and 7 in aqueous solution. The metal ion investigated were: Cu(II), Co(II), Cr(III), Ni(II), Cd(II), Zn(II), and Fe(III). The polymeric systems showed high metal ion retention for Zn (II) and Fe(III) at different pH. At different pHs, the MRC of the poly(MG-co-CA) for Fe(III) ions varied from 122.1 to 146.2 mg/g and from 120.5 to 133.5 mg/g, (samples 1 and 2 at pH 3 and 7, respectively). The MRC had the highest retention values for both copolymer systems at pH 7. The copolymers presented higher thermal decomposition temperature (TDT) in comparison with copolymer–metal complexes at pH 3 and 5. The cyclic voltammetry (CV) for poly(MG-co-CA) (20 mM) was compared with the CV of the [poly(MG-co-CA)–Fe(III)] copolymer complex. Moreover, [poly(MG-co-CA)–Fe(III)] showed a redox wave difference between +0.25 and +0.50 V possibly due to the presence of metal complexed with the polymer. The electrochemical characterization of the copolymer poly(MG-co-AC) shown the reduction of carboxylic acid groups of the N-maleoylglycine and crotonic acid moiety to hydroxyl group. The results support the assumption that the copolymer presents convenient electroactivity.











Similar content being viewed by others
References
Rivas BL, Pereira E, Cid R, Geckeler KE (2005) Polyelectrolyte-assisted removal of metal ions with ultrafiltration. J Appl Polym Sci 95(5):1091–1099
Saglam A, Bektas S, Patir S, Genc Ö, Denizli A (2001) Novel metal complexing ligand: thiazolidine carrying poly(hydroxyethylmethacrylate) microbeads for removal of cadmium (II) and lead (II) ions from aqueous solutions. React Funct Polym 47(3):185–192
Denizli A, Kesenci K, Salih B, Senel S, Piskin E (1999) Metal chelating properties of Cibacron Blue F3GA-derived poly(EGDMA-HEMA) microbeads. J Appl Polym Sci 71(9):13971403
Moreno-Villoslada I, Rivas BL (2003) Retention of metal ions in ultrafiltration of mixtures of divalent metal ions and water-soluble polymers at constant ionic strength based on Freundlich and Langmuir isotherms. J Membr Sci 215(1–2):195–202
Beatty ST, Fischer RJ, Hagers DL, Rosenberg E (1999) A comparative study of the removal of heavy metal ions from water using a silica-polyamine composite and a polystyrene chelator resin. Ind Eng Chem Res 38(11):4402–4408
Li W, Zhao H, Teasdale PR, John R, Zhang S (2002) Synthesis and characterisation of a polyacrylamide-polyacrylic acid copolymer hydrogel for environmental analysis of Cu and Cd. React Funct Polym 52(1):31–41
Rivas BL, Pooley SA, Luna M (2000) Chelating properties of poly(N-acryloyl piperazine) by liquid-phase polymer-based retention (LPR) technique. Macromol Rapid Commun 21(13):905–908
Rivas BL, Moreno-Villoslada I (2001) Polyelectrolyte behavior of three copolymers of 2-acrylamido-2-methyl-propanesulfonic acid and N-acryloyl-N’-methylpiperazine studied by ultrafiltration. J Membr Sci 187(1–2):271–275
Rivas BL, Maturana HA, Villegas S (2000) Adsorption behavior of metal ions by amidoxime chelating resin. J Appl Polym Sci 77(9):1994–1999
Rzaev ZMO (2000) Complex-radical alternating copolymerization. Progr Polym Sci 25(2):163–217
Morlay C, Cromer M, Mouginot M, Vittori O (1999) Potentiometric study of Cd(II) and Pb(II) complexation with two high molecular weight poly(acrylic acids); comparison with Cu(II) and Ni(II). Talanta 48(5):1159–1166
Bharel R, Choudhary V, Varna IK, Wang FW (1995) Physicomecanical properties of poly(methyl methacrylate-co-N-arylmaleimides). J Appl Polym Sci 57(6):767–773
Choudhary V, Mishra A (1996) Studies on the copolymerization of methyl methacrylate and N-arylmaleimides. J Appl Polym Sci 62(4):707–712
Morlay C, Cromer M, Vittori O (2000) The removal of copper(II) and nickel (II) from dilute aqueous solution by a synthetic flocculant: a polarographic study of the complexation with a high molecular weight poly(acrylic acid) for different pH value. Water Res 34(2):455–462
Mouginot Y, Morlay C, Cromer M, Vittori O (2000) Potentiometric study of copper (II) and nickel (II) complexation by a cross-linked poly(acrylic acid) gel. Anal Chim Acta 407(1–2):337–345
Disbudak A, Bektas S, Patir S, Genc Ö, Denizli A (2002) Cysteine-metal affinity chromatography: determination of heavy metal adsorption properties. Sep Purif Technol 26(2–3):273–281
del Pizarro GC, Marambio OG, Jeria-Orell M, Huerta MR, Rodríguez OO, Rivas BL, Geckeler KE (2007) Metal-ion interaction of water-soluble copolymers containing carboxylic acid groups in aqueous phase by membrane filtration technique. J Appl Polym Sci 105(5):2893–2902
Rivas BL, del Pizarro GC, Marambio OG, Geckeler KE (1998) Thermal properties of poly(maleyl glycine) and poly(maleyl glycine-co-acrylic acid) and their metal complexes. Polym Bull 41:317–324
del Pizarro GC, Marambio OG, Jeria-Orell M, Oyarzún DP, Rivas BL, Habicher WD (2007) Preparation, characterization, and metal ion retention capacity of Co(II) and Ni(II) from poly(p-HO- and p-Cl-phenyl maleimide-co-2-hydroxypropylmetha crylate using the ultra filtration technique. J Appl Polym Sci 106:2448–2455
Sohn BH, Cohen RE (1997) Processible optically transparent block copolymer films containing superparamagnetic iron oxide manoclusters. Chem Mater 9:264–269
Ali HA, Iliadis AA (2004) Comparative study of self-assembled ZnO nanostructures in poly(styrene-acrylic acid) diblock copolymers-[PS]m[PAA]n on Si and SiO2/Si surfaces. Thin Solid Films 469–470, 425–430
Borah HN, Boruah RC, Sandhu JS (1998) Microware-induced one-pot synthesis of N-carboxyalkylmaleimides and phthalimides. J Chem Res S(1):272–273
del Pizarro GC, Rivas BL, Geckeler KE (1997) Preparation and characterization of water-soluble poly(acrylic-co-n-maleylglycine). J Macromol Sci Pure Appl Chem A34(5):855–864
Marambio OG, Pizarro GD, Jeria-Orell M, Huerta M, Olea-Azar C, Habicher WD (2005) Poly(N-phenylmaleimide-co-acrylic acid)-copper (II) and Poly(N-phenylmaleimide-co-acrylic acid)-cobalt (II) complexes: synthesis, characterization, and thermal behavior. J Polym Sci Part A Polym Chem 43(20):4933–4941
Rivas BL, Schiappacasse LN, Pereira E, Moreno-Villoslada I (2004) Error simulation in the determination of the formation constant of polymer-metal complexes (PMC) by liquid-phase polymer-based retention (LPR) technique. J Chil Chem Soc 49(4):345–350
Rivas BL, Pereira ED, Gallegos P, Geckeler KE (2002) Water-soluble acidic polyelectrolytes with metal removing ability. Polym Adv Technol 13:10–12
Moreno-Villoslada I, Rivas BL (2002) Competition of divalent metal ions with monovalent metal ions on the adsorption on water-soluble polymer. J Phys Chem B 106(38):9708–9711
del Pizarro GC, Rivas BL, Geckeler KE (1996) Metal complexing properties of a water-soluble poly(n-maley glycine) studied by liquid-phase polymer-based retention technique. Polym Bull 37(4):525–530
Tuchida E, Abe K (1982) Interactions between macromolecules in solution and intermacromolecular complexes. Adv Polym Sci 45:1–91
Lekchiri A, Castello A, Morcellet J, Morcellet M (1991) Copper-complexes and catalase-like activity of a polyelectrolyte derived from aspartic acid. Eur Polym J 27(11):1271–1278
Choi SJ, Geckeler KE (2000) Synthesis and properties of hydrophilic polymer. Part 8. Preparation, characterization and metal complexing of poly[(2-hydroxyethyl)-DL-aspartamide] in aqueous solution. Polym Int 49(11):1519–1524
Acknowledgments
The authors thank the Dirección de Investigación de la Universidad Tecnólogica Metropolitana (Project UTEM 293/07, 294/07), Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ), and the Deutsche Akademische Austauschdienst (DAAD) for financial support. Rivas BL thanks CIPA the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marambio, O.G., Sánchez, J., del C. Pizarro, G. et al. Free radical copolymerization of functional water-soluble poly(N-maleoylglycine-co-crotonic acid): polymer metal ion retention capacity, electrochemical, and thermal behavior. Polym. Bull. 65, 701–717 (2010). https://doi.org/10.1007/s00289-010-0298-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-010-0298-6