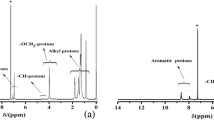
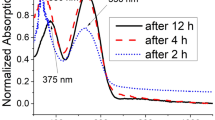
Abstract
New through-space conjugated polymers comprising the pseudo-ortho-linked [2.2]paracyclophane moiety were synthesized by the Sonogashira coupling reaction. All the synthesized polymers were soluble in common organic solvents and could form thin films. The UV–vis absorption spectra of the synthesized polymers revealed an extension of the conjugation length owing to the through-space interactions. The polymers exhibited a blue-light emission in both solution and film states.






Similar content being viewed by others
References
Brown CJ, Farthing AC (1949) Preparation and structure of di-p-xylylene. Nature 164:915–916
Cram DJ, Allinger NL, Steinberg H (1954) Macro rings. 7. The spectral consequences of bringing 2 benzene rings face to face. J Am Chem Soc 76:6132–6141
Vögtle F (ed) (1994) Cyclophanes. Top Curr Chem 115
Canuto S, Zerner MC (1990) Theoretical interpretation of the absorption and ionization spectra of the paracyclophanes. J Am Chem Soc 112:2114–2120
Yamakita Y, Yamauchi M, Ohno K (2000) Penning ionization of [2,2]-paracyclophane by collision with metastable He*(23S) atoms. Chem Phys Lett 322:189–198
Oldham WJ Jr, Miao YJ, Lachicotte RJ, Bazan GC (1998) Stilbenoid dimers: effect of conjugation length and relative chromophore orientation. J Am Chem Soc 120:419–420
Bazan GC, Oldham WJ Jr, Lachicotte RJ, Tretiak S, Chernyak V, Mukamel S (1998) Stilbenoid dimers: dissection of a paracyclophane chromophore. J Am Chem Soc 120:9188–9204
Wang S, Bazan GC, Tretiak S, Mukamel S (2000) Oligophenylenevinylene phane dimers: probing the effect of contact site on the optical properties of bichromophoric pairs. J Am Chem Soc 122:1289–1297
Vögtle F (1993) Cyclophane chemistry. Wiley, New York
Weber E (ed) (1994) Cyclophanes. Top Curr Chem 172
Cleiter R, Hopf H (eds) (2004) Modern cyclophane chemistry. Wiley, Weinheim
Salhi F, Lee B, Metz C, Bottomley LA, Collard DM (2002) Influence of π-stacking on the redox properties of oligothiophenes: (α-alkyloligo-thienyl)para[2.2]cyclophanes. Org Lett 4:3195–3198
Salhi F, Collard DM (2003) π-Stacked conjugated polymers: the influence of paracyclophane π-stacks on the redox and optical properties of a new class of broken conjugated polythiophenes. Adv Mater 15:81–85
Guyard L, Audebert P (2001) Synthesis and electrochemical polymerization of bis-dithienyl cyclophane. Elecrochem Commun 3:164–167
Guyard L, Audebert P, Dolbier WR Jr, Duan J-X (2002) Synthesis and electrochemical polymerization of new oligothiophene functionalized fluorocyclophanes. J Electroanal Chem 537:189–193
Morisaki Y, Chujo Y (2006) Through-space conjugated polymers based on cyclophanes. Angew Chem Int Ed 45:6430–6437
Morisaki Y, Chujo Y (2008) Cyclophane-containing polymers. Prog Polym Sci 33:346–364
Morisaki Y, Chujo Y (2002) Synthesis of novel π-conjugated polymers having [2.2]paracyclophane skeleton in the main chain. Extension of π-conjugated length via the through-space. Macromolecules 35:587–589
Morisaki Y, Chujo Y (2002) Synthesis of novel alternating π-conjugated copolymers having [2.2]paracyclophane and fluorene units in the main chain leading to the blue light-emitting materials. Chem Lett 194–195
Morisaki Y, Ishida T, Chujo Y (2002) Synthesis and properties of novel through-space π-conjugated polymers based on poly(p-phenylenevinylene)s having a [2.2]paracyclophane skeleton in the main chain. Macromolecules 35:7872–7877
Morisaki Y, Chujo Y (2002) Synthesis and optical properties of the [2.2]paracyclophane-containing π-conjugated polymer with a diacetylene unit. Polym Bull 49:209–215
Morisaki Y, Fujimura F, Chujo Y (2003) Synthesis and properties of novel σ-π-conjugated polymers with alternating organosilicon and [2.2]paracyclophane units in the main chain. Organometallics 22:3553–3557
Morisaki Y, Chujo Y (2003) Synthesis and properties of a novel through-space conjugated polymer with [2.2]paracyclophane and ferrocene in the main chain. Macromolecules 36:9319–9324
Morisaki Y, Chujo Y (2004) Novel [2.2]paracyclophane-fluorene-based conjugated copolymers: synthesis, optical, and electrochemical properties. Macromolecules 37:4099–4103
Morisaki Y, Ishida T, Tanaka H, Chujo Y (2004) Synthesis and properties of the [2.2]paracyclophane-containing conjugated polymer using benzothiadiazole as an electron accepter. J Polym Sci Part A Polym Chem 42:5891–5899
Morisaki Y, Wada N, Chujo Y (2005) Novel conjugated polymers containing [2.2]paracyclophane and carbazole units with efficient photoluminescence. Polym Bull 53:73–80
Morisaki Y, Wada N, Chujo Y (2005) Novel π-conjugated cyclophane polymers containing phenylamine moieties with strong blue-light emission. Polymer 46:5884–5889
Morisaki Y, Chujo Y (2005) Novel through-space conjugated polymers consisting of alternate [2.2]paracyclophane and fluorene. Bull Chem Soc Jpn 78:288–293
Morisaki Y, Chujo Y (2005) Construction of benzene ring-layered polymers. Tetrahedron Lett 46:2533–2537
Morisaki Y, Murakami T, Chujo Y (2008) Synthesis and properties of [2.2]paracyclophane-layered polymers. Macromolecules 41:5960–5963
Reich HJ, Cram DJ (1969) Macro rings. XXXVI. Ring expansion, racemization, and isomer interconversions in the [2.2]paracyclophane system through a diradical intermediate. J Am Chem Soc 91:3517–3526
Pye PJ, Rossen K, Reamer RA, Tsou NN, Volante RP, Reider PJ (1997) A new planar chiral bisphosphine ligand for asymmetric catalysis: highly enantioselective hydrogenations under mild conditions. J Am Chem Soc 119:6207–6208
Braddock DC, MacGilp ID, Perry BG (2002) Improved synthesis of (+/−)-4,12-dihydroxy[2.2]paracyclophane and its enantiomeric resolution by enzymatic methods: planar chiral (R)- and (S)-phanol. J Org Chem 67:8679–8681
Bondarenko L, Dix I, Hinrichs H, Hopf H (2004) Cyclophanes. Part LII: Ethynyl[2.2]paracyclophanes—new building blocks for molecular scaffolding. Synthesis 16:2751–2759
Pangborn AB, Giardello MA, Grubbs RH, Rosen RK, Timmers FJ (1996) Safe and convenient procedure for solvent purification. Organometallics 15:1518–1520
Wolkoff P (1975) New method of preparing hydrazonyl halides. Can J Chem 53:1333–1335
Li H, Powell DR, Hayashi RK, West R (1998) Poly((2,5-dialkoxy-p-phenylene)ethynylene-p-phenyleneethynylene)s and their model compounds. Macromolecules 31:52–58
Acknowledgments
This work was supported by Grant-in-Aid for Creative Scientific Research of “Invention of Conjugated Electronic Structures and Novel Functions”, No. 16GS0209, from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Morisaki, Y., Wada, N., Arita, M. et al. Synthesis of through-space conjugated polymers containing the pseudo-ortho-linked [2.2]paracyclophane moiety. Polym. Bull. 62, 305–314 (2009). https://doi.org/10.1007/s00289-008-0021-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-008-0021-z