Summary
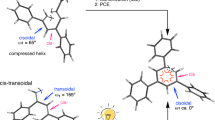
The backbone of polyethers are often flexible because of inherent characteristics of ether linkage, but polyethers from chiral epoxides could form stable one-handed helical structure in solution state, if the pendants on the chain of polyethers are enough bulky and the distance between pendants and main chain is appropriate.
Similar content being viewed by others
References
Watson JD, Crick FHC (1953) Nature 171:737
Okamato Y, Honda S, Okamato H, Yuki S, Murata S, Noyori S, Takaya H (1981) J Am Chem Soc 103:6971
Okamoto Y, Adachi M, Shohi H, Yuki H (1981) Polym Journal 13:175
Corley LS, Vogl O (1980) Polym Bull 3:211
Millich F, Baker G K (1969) Macromolecules 2:122
Patten T, Novak BM (1991) J Am Chem Soc 113:5065
Krishnan M, Balasubramanian S (2004) Chemical Phsyics Letter 391:200
Yang R, Yang XR, Evans DF, Hendrickson WA, Baker J (1990) Journal of Phsyscial Chemistry 94:6123
Nakano T, Okamato Y (2001) Chem Rev 107:1013
Price CC (1974) Acc Chem Res 7:294
Lok CM (1978) Chemistry and Physics of Lipids 22:323
Grovenstein E, Cottingham AB, Gelbaum LT (1978) J Org Chem 43:3332
Eßwein B, Molenberg A, Möller M (1996) Macromol. Symp 107:331
Optically active 4,4,4-triphenyl-1,2-butanediol, 1HNMR (CDCl3): δ=7.18-7.37 (m, 15H, PhH), 3.75-3.80(m, 1H, - CH-), 3.14 (dd, J1-4.1Hz, J2=14.8Hz 1H,- CH2-), 3.01-3.05 (m, 1H, -CH2-), 2.82-2.86 (m, 1H, -CH2-), 2.67 (dd, J1-5.2 Hz, J2=14.8Hz, 1H, -CH2-CPh3), 1.86 (m, 12H, -OH), 1.25 (m, 1H, -OH); 13CNMR(CDCl3): δ= 146.63, 129.02, 128.21, 126.36, 69.46, 56.05, 50.88, 45.13. [α]58920= -38o (c 0.2, THF)
Optically active 4,4,4-triphenyl-1-tert-butoxyl-2-butanol, 1HNMR (CDCl3): δ=7.12-7.35 (m, 15H, PhH), 3.61-3.64 (m, 1H, - CH-), 2.99 (dd, 1H, J1=2.8Hz, J2=14.7Hz, -CH2-CPh3), 2.76-2.81 (m, 1H, -CH2-), 2.54 (dd, 1H, J=5.3Hz, J=14.6Hz, -CH2-CPh3), 2.36 (dd, 1H, m, 1H, -CH2-), 2.16 (m, 1H, -OH), 0.98 (s, 9H, -C(CH3)3); 13CNMR(CDCl3): δ= 146.26, 128.92, 128.33, 126.15, 69.22, 68.13, 55.92, 50.35.88, 45.13, 29.56.[α]58920 = -37o (c 0.2, THF)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, J., Yang, NF., Li, JC. et al. Helical Polymers from Optically Active Epoxides with Bulky Pendants. Polym. Bull. 59, 481–490 (2007). https://doi.org/10.1007/s00289-007-0788-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-007-0788-3