Summary
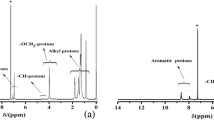
Novel π-conjugated polymers 10a-c having the phosphole ring were prepared by PdCl2(PPh3)2/CuI catalyzed coupling of 1-phenyl-2,5-bis(p-bromophenyl)phosphole 8 with fluorine-substituted diethynylbenzene 9a-c. The obtained polymers 10a-c were regio-regulated with the 2,5-substituted phosphole ring in the polymer main chain, and characterized by 1H, 31P NMR, and FTIR. Polymers 10a-c were found to be an extended π-conjugated system according to the results of UV-Vis absorption spectra. In the fluorescence emission spectra of 10a-c, moderate emission peaks were observed in the visible blue to green region.
Similar content being viewed by others
References and Notes
(a) Krft A, Grimsdale AC, Holmes AB (1998) Angew Chem Int Ed 37:403 (b) van Hutten PF, Wildeman J, Meetama A, Hadziioannou G (1999) J Am Chem Soc 121:5910 (c) Mitschke U, Bäuerle P (2000) J Mater Chem 10:1471 (d) Greiner A, Weder C (2003) Light-emitting diodes. In: Kroschwitz JI (ed) Encyclopedia of polymer science and technology, 3rd edn, vol. 3. Wiley-Interscience, New York
(a) Horowitz G (1998) Adv Mater 10:365 (b) Müllen K, Wegner G (1998) Electronic Materials: The oligomer Approach, Wiley-VCH, Weinheim
McQuade DT, Pullen AE, Swager TM (2000) Chem Rev 100:2537
Skothim TA, Elsenbaumer RL, Reynolds J, editors (1998) Handbook of Conducting Polymers, 2nd ed, Marcel Dekker, New York
Organophosphorus conjugated materials were reviewed; Baumgartner T, Réau R (2006) Chem Rev 106:4681
Phosphole-containing conjugated oligomers were reported; (a) Bévierr MO, Mercier F, Ricard L, Mathey F (1990) Angew Chem Int Ed Engl 29:655 (b) Bévierre MO, Mercier F, Ricard L, Mathey F, Jutand A, Amatore CN (1991) New J Chem 15:545 (c) Deschamps E, Ricard L, Mathey F (1994) Angew Chem Int Ed Engl 33:1158
(a) Hay C, Le Vilain D, Deborde V, Toupet L, Réau R (1999) Chem Commun 345 (b) Hay C, Fischmeister C, Hissler M, Toupet L, Réau R (2000) Angew Chem Int Ed 10:1812 (c) Hay C, Hissler M, Fischmeister C, Rault-Berthelot J, Toupet L, Nyulászi L, Réau R (2001) Chem Eur J 7:4222 (d) Fave C, Hissler M, Sénéchal K, Ledoux I, Zyss J, Réau R (2002) Chem Commun 1674 (e) Hay C, Fave C, Hissler M, Rault-Berthelot J, Réau R (2003) Org Lett 19:3467 (f) Fave C, Hissler M, Karpati T, Rault-Berthelot J, Deborde V, Toupet L, Nyulászi L, Réau R (2004) J Am Chem Soc 126:6058
(a) Quin LD, Quin GS (2001) In Phosphorus-Carbon Heterocyclic Chemistry: The Rise of a New Domain, Mathey F, editor, Pergamon, Amsterdam (b) Dillon KB, Mathy F, Nixon JF (1998) Phosphorus, The Carbon Copy, Wiley, Chichester (c) Quin LD (1996) In Comprehensive Heterocyclic Chemistry, vol 2, Katritzky AR, Ress CW, Scriven EFV, editors, Elsevier, Oxford
Wittig G, Geissler G (1953) Justus Liebigs Ann Chem 580:4
(a) Leavitt FC, Manuel TA, Johnson F (1959) J Am Chem Soc 81:3163 (b) Braye EH, Hübel W (1959) Chem Ind 1250
Mao SSH, Tilley TD (1997) Macromolecules 30:5566
Morisaki Y, Aiki , Chujo Y (2003) Macromolecules 36:2594
(a) Fave C, Cho TY, Hissler M, Chen CW, Luh, TY, Wu CC, Réau R (2003) J Am Chem Soc 125:9254 (b) Su HC, Fadhel O, Yang CJ, Cho TY, Fave C, Hisller M, Wu CC, Réau R (2006) J Am Chem Soc 128:983
Khan MS, Al-Mandhary MRA, Al-Suti MK, Corcoran TC, Al-Mahrooqi Y, Attfield JP, Feeder N, David WIF, Shankland K, Friend RH, Köhler A, Marseglia EA, Tedesco E, Tang CC, Raithby PR, Collings JC, Roscoe KP, Batsanov AS, Stimson LM, Marder TB (2003) New J Chem 27:140
Sonogashira K, Tohda Y, Hagihara N (1975) Tetrahedron Lett 16:4467
(a) Negishi E, Cederbaum FE, Takahashi T (1986) Tetrahedron Lett 27:2829 (b) Negishi E, Takahashi T (1994) Acc Chem Res 27:124
(a) Doherty S, Robins EG, Nieuwenhuyzen M, Knight JG, Champkin PA, Clegg W (2002) Organometallics 21:1383 (b) Doherty S, Knight JG, Robins EG, Scanlan TH, Champkin PA, Clegg W (2001) J Am Chem Soc 123:5110 (c) Takahashi T, Sun WH, Nakajima K (1999) Chem Commun 1595
The absorbance of each sample was below 0.05 at the excitation wavelength at 350 nm, in the measurement of the fluorescence quantum yield. The quantum yield (Φunk) of unknown sample was calculated by the following equation: Φunk = Φstd[AstdFunk/AunkFstd][nD,unk/nD,std]2 where Astd and Aunk are the absorbance of the standard and unknown sample, respectively, Fstd and Funk are the corresponding relative integrated fluorescence intensities, and nD is the refractive index [CH2Cl2 (nD=1.424) and CHCl3 (nD=1.446) were used].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morisaki, Y., Na, HS., Aiki, Y. et al. Synthesis and Characterization of π-Conjugated Polymers with a 2,5-Substituted Phosphole Skeleton. Polym. Bull. 58, 777–784 (2007). https://doi.org/10.1007/s00289-007-0725-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-007-0725-5