Abstract
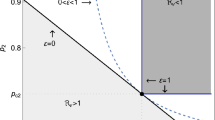
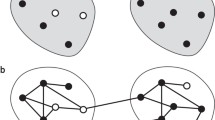
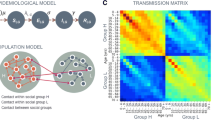
Randomized trials of infectious disease interventions, such as vaccines, often focus on groups of connected or potentially interacting individuals. When the pathogen of interest is transmissible between study subjects, interference may occur: individual infection outcomes may depend on treatments received by others. Epidemiologists have defined the primary parameter of interest—called the “susceptibility effect”—as a contrast in infection risk under treatment versus no treatment, while holding exposure to infectiousness constant. A related quantity—the “direct effect”—is defined as an unconditional contrast between the infection risk under treatment versus no treatment. The purpose of this paper is to show that under a widely recommended randomization design, the direct effect may fail to recover the sign of the true susceptibility effect of the intervention in a randomized trial when outcomes are contagious. The analytical approach uses structural features of infectious disease transmission to define the susceptibility effect. A new probabilistic coupling argument reveals stochastic dominance relations between potential infection outcomes under different treatment allocations. The results suggest that estimating the direct effect under randomization may provide misleading conclusions about the effect of an intervention—such as a vaccine—when outcomes are contagious. Investigators who estimate the direct effect may wrongly conclude an intervention that protects treated individuals from infection is harmful, or that a harmful treatment is beneficial.



Similar content being viewed by others
Data availability
All data and code are available upon request.
References
Acosta CJ, Galindo CM, Mohammad A, Abu ER, Leon OR, Carolina D-HM, Anne-Laure P, Dinh TV, Yang J, Kyung PJ et al (2005) A multi-country cluster randomized controlled effectiveness evaluation to accelerate the introduction of Vi polysaccharide typhoid vaccine in developing countries in Asia: rationale and design. Trop Med Int Health 10(12):1219–1228
Aiello AE, Simanek AM, Eisenberg MC, Walsh AR, Davis B, Volz E, Cheng C, Rainey JJ, Uzicanin A, Gao H et al (2016) Design and methods of a social network isolation study for reducing respiratory infection transmission: the eX-FLU cluster randomized trial. Epidemics 15:38–55
Anderson RM, May RM (1992) Infectious disease of humans, dynamics and control. Oxford University Press, Oxford
Andersson H, Britton T (2000) Stochastic epidemic models and their statistical analysis. Springer, New York
Auranen K, Arjas E, Leino T, Takala AK (2000) Transmission of pneumococcal carriage in families: a latent Markov process model for binary longitudinal data. J Am Stat Assoc 95:1044–1053
Becker Niels G (1989) Analysis of infectious disease data. Chapman and Hall London, London
Becker NG, Britton T (2004) Estimating vaccine efficacy from small outbreaks. Biometrika 91(2):363–382
Becker NG, Britton T, O’Neill PD (2003) Estimating vaccine effects on transmission of infection from household outbreak data. Biometrics 59(3):467–475
Becker NG, Britton T, O’Neill PD (2006) Estimating vaccine effects from studies of outbreaks in household pairs. Stat Med 25(6):1079–1093
Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, Bernstein DI, Hayden FG, Kotloff K, Zangwill K et al (1998) The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine in children. New England J Med 338(20):1405–1412
Britton T, Lindenstrand D (2009) Epidemic modelling: aspects where stochasticity matters. Math Biosci 222(2):109–116
Buchanan AL, Vermund SH, Friedman SR, Spiegelman D (2018) Assessing individual and disseminated effects in network-randomized studies. Am J Epidemiol 187(11):2449–2459
Cai X, Loh WW, Crawford FW (2021) Identification of causal intervention effects under contagion. J Causal Inference 9(1):9–38
Cauchemez S, Carrat F, Viboud C, Valleron AJ, Boëlle PY (2004) A Bayesian MCMC approach to study transmission of influenza: application to household longitudinal data. Stat Med 23:3469–87
Cauchemez S, Temime L, Guillemot D, Varon E, Valleron A-J, Thomas G, Boëlle P-Y (2006) Investigating heterogeneity in pneumococcal transmission: a Bayesian MCMC approach applied to a follow-up of schools. J Am Stat Assoc 101(475):946–958
Cauchemez S, Donnelly CA, Reed C, Ghani AC, Fraser C, Kent CK, Finelli L, Ferguson NM (2009) Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med 361(27):2619–2627
Cole SR, Lau B, Eron JJ, Brookhart MA, Kitahata MM, Martin JN, Mathews WC, Mugavero MJ (2015) Estimation of the standardized risk difference and ratio in a competing risks framework: application to injection drug use and progression to AIDS after initiation of antiretroviral therapy. Am J Epidemiol 181(4):238–245
den Hollander F (2012) Probability theory: the coupling method. Mathematical Institute, Leiden University. URL https://urldefense.com/v3/http://websites.math.leidenuniv.nl/probability/lecturenotes/CouplingLectures.pdf__;!!DZ3fjg!8heTQp1C9N_G5yKAN0uE4UEfx8a8n9_NGHnVMJSbx0LxNSGsbd7qEkuuNvnIhGLbrMG4_4pD6B2WOLlp1QiG5g
Devroye L (1986) Non-uniform random variate generation. Springer, Cham
Diekmann O, Heesterbeek H, Britton T (2012) Mathematical tools for understanding infectious disease dynamics. Princeton University Press, Princeton
Greenland S, Robins JM (1986) Identifiability, exchangeability, and epidemiological confounding. Int J Epidemiol 15(3):413–419
Greenwood M, Yule GU (1915) The statistics of anti-typhoid and anti-cholera inoculations, and the interpretation of such statistics in general
Elizabeth Halloran M, Struchiner CJ (1995) Causal inference in infectious diseases. Epidemiology 6:142–151
Halloran ME, Haber M, Longini IM, Struchiner CJ (1991) Direct and indirect effects in vaccine efficacy and effectiveness. Am J Epidemiol 133(4):323–331
Elizabeth Halloran M, Struchiner Claudio J, Longini IM Jr (1997) Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am J Epidemiol 146(10):789–803
Elizabeth Halloran M, Longini Ira M, Struchiner Claudio J (2010) Design and analysis of vaccine studies. Springer, Cham
Hayden FG, Gubareva LV, Monto AS, Klein TC, Elliott MJ, Hammond JM, Sharp SJ, Ossi MJ (2000) Inhaled zanamivir for the prevention of influenza in families. New England J Med 343(18):1282–1289
Hudgens MG, Halloran ME (2008) Toward causal inference with interference. J Am Stat Assoc 103(482):832–842
Karwa V, Airoldi EM (2018) A systematic investigation of classical causal inference strategies under mis-specification due to network interference. arXiv preprint arXiv:1810.08259
Kaslow DC (2002) Transmission-blocking vaccines. Malaria Immunol 80:287–307
Kenah E (2013) Non-parametric survival analysis of infectious disease data. J R Stat Soc B 75(2):277–303
Kenah E (2014) Semiparametric relative-risk regression for infectious disease transmission data. J Am Stat Assoc 110:313–325
Longini IM Jr, Hudgens MG, Halloran ME, Sagatelian K (1999) A Markov model for measuring vaccine efficacy for both susceptibility to infection and reduction in infectiousness for prophylactic HIV vaccines. Stat Med 18(1):53–68
Monto AS, Pichichero ME, Blanckenberg SJ, Ruuskanen O, Cooper C, Fleming DM, Kerr C (2002) Zanamivir prophylaxis: an effective strategy for the prevention of influenza types A and B within households. J Infect Dis 186(11):1582–1588
Morozova O, Cohen T, Crawford FW (2018) Risk ratios for contagious outcomes. J R Soc Interface 15(138):20170696
O’Hagan JJ, Lipsitch M, Hernán MA (2014) Estimating the per-exposure effect of infectious disease interventions. Epidemiology 25(1):134–138
O’Neill PD, Balding DJ, Becker NG, Eerola M, Mollison D (2000) Analyses of infectious disease data from household outbreaks by Markov chain Monte Carlo methods. J R Stat Soc Ser C 49(4):517–542
Rhodes PH, Halloran ME, Longini IM Jr (1996) Counting process models for infectious disease data: distinguishing exposure to infection from susceptibility. J R Stat Soc B 58:751–762
Ross SM (1996) Stochastic processes. Wiley, New York
Rothman KJ, Greenland S, Lash TL (2008) Modern epidemiology. Lippincott Williams & Wilkins, Philadephia
Rubin DB (2005) Causal inference using potential outcomes: design, modeling, decisions. J Am Stat Assoc 100(469):322–331
Sävje F, Aronow PM, Hudgens MG (2021) Average treatment effects in the presence of unknown interference. Ann Stat 49(2):673–701
Simondon F, Preziosi M-P, Yam A, Kane CT, Chabirand L, Iteman I, Sanden G, Mboup S, Hoffenbach A, Knudsen K et al (1997) A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine 15(15):1606–1612
Sobel ME (2006) What do randomized studies of housing mobility demonstrate? Causal inference in the face of interference. J Am Stat Assoc 101(476):1398–1407
Struchiner CJ, Halloran ME (2007) Randomization and baseline transmission in vaccine field trials. Epidemiol Infect 135(2):181–194
Tsang TK, Cowling BJ, Fang VJ, Chan K-H, Dennis KM, Leung GM, Peiris JSM, Cauchemez S (2015) Influenza A virus shedding and infectivity in households. J Infect Dis 212(9):1420–1428
Tsang TK, Fang VJ, Chan K-H, Dennis KM, Leung GM, Peiris JSM, Cowling BJ, Cauchemez S (2016) Individual correlates of infectivity of influenza A virus infections in households. PloS One 11(5):e0154418
van Boven M, Ruijs WLM, Wallinga J, O’Neill PD, Hahne S (2013) Estimation of vaccine efficacy and critical vaccination coverage in partially observed outbreaks. PLoS Comput Biol 9(5):e1003061
Vander Weele TJ, Robins JM (2012) Stochastic counterfactuals and stochastic sufficient causes. Stat Sin 22(1):379
Vander Weele TJ, Tchetgen EJT (2011) Effect partitioning under interference in two-stage randomized vaccine trials. Stat Prob Lett 81(7):861–869
Welliver R, Monto AS, Carewicz O, Schatteman E, Hassman M, Hedrick J, Jackson HC, Huson L, Ward P, Oxford JS et al (2001) Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. J Am Med Assoc 285(6):748–754
Acknowledgements
We are especially grateful to Michael Hudgens and M. Elizabeth Halloran for helpful comments. In addition, we thank P. M. Aronow, Xiaoxuan Cai, Ted Cohen, Soheil Eshghi, Gregg S. Gonsalves, Eben Kenah, Zehang Li, Wen Wei Loh, Sida Peng, Fredrik Sävje, Yushuf Sharker, and Daniel Weinberger for helpful comments. This work was supported by NIH grants NICHD DP2 HD091799-01 and NIDA R36 DA042643.
Funding
This work was supported by NIH grants NICHD DP2 HD091799-0 and NIDA R36 DA042643.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors claim no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eck, D.J., Morozova, O. & Crawford, F.W. Randomization for the susceptibility effect of an infectious disease intervention. J. Math. Biol. 85, 37 (2022). https://doi.org/10.1007/s00285-022-01801-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-022-01801-8