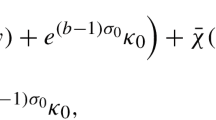Abstract
Although macrophages are part of the human immune system, it has been remarkably observed in laboratory experiments that decreasing its number can slow down the tumor progression. We analyze through a recently mathematical model proposed in the literature, necessary conditions for aggregation of tumor cells and macrophages. In order to do so, we prove the possibility of having blow-up in finite time. Next, we study if the aggregation of macrophages can occur when having a low density of tumor cells, and vice versa. With this purpose, we consider the problem of analyzing the existence or not of a simultaneous blow-up. We achieve this goal thanks to a novel process that allows us to compare the entropy functional associated with the density of each population, which turns out to be also a method to find enough conditions for having a simultaneous blow-up.
Similar content being viewed by others
References
Aubin JP (1963) Un théoreme de compacité. CR Acad Sci Paris 256(24):5042–5044
Benguria R, Brézis H, Lieb EH (1981) The Thomas–Fermi–von Weizsacker theory of atoms and molecules. Commun Math Phys 79(2):167–180
Blanchet A, Carrillo JA, Masmoudi N (2008) Infinite time aggregation for the critical Patlak–Keller–Segel model in R2. Commun Pure Appl Math 61(10):1449–1481
Blanchet A, Dolbeault J, Perthame B (2006) Two-dimensional Keller–Segel model: optimal critical mass and qualitative properties of the solutions. Electron J Differ Equ 44:1–32
Calvez V (2007) Mathematical models and analysis for the collective motion of cells. Ph.D. thesis, Department of Mathematics, University of Paris 6 and Ecole Normale Superieure, Paris
Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L (2010) Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78(9):838–848
Carlen E, Loss M (1992) Competing symmetries, the logarithmic HLS inequality and Onofri’s inequality ons\(^{n}\). Geom Funct Anal GAFA 2(1):90–104
Conca C, Espejo E, Vilches K (2011) Remarks on the blowup and global existence for a two species chemotactic Keller–Segel system in \({\mathbb{R}}^{2}\). Eur J Appl Math. https://doi.org/10.1017/S0956792511000258
Condeelis J, Segall JE (2003) Intravital imaging of cell movement in tumours. Nat Rev Cancer 3(12):921
Condeelis J, Pollard JW (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124(2):263–266
Corrias L, Perthame B (2008) Asymptotic decay for the solutions of the parabolic-parabolic Keller–Segel chemotaxis system in critical spaces. Math Comput Modell 47(7):755–764
Espejo E, Vilches K, Conca C (2013) Sharp condition for blow-up and global existence in a two species chemotactic Keller–Segel system in \({\mathbb{R}}^{2}\). Eur J Appl Math 24:297–313
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3(5):362
Fernández GE, Mischler S (2016) Uniqueness and long time asymptotic for the Keller–Segel equation: the parabolic–elliptic case. Arch Ration Mech Anal 220(3):1159–1194
Fonseca I, Leoni G (2007) Modern methods in the calculus of variations: \(L^{p}\) spaces. Springer, Berlin
Hernandez L, Smirnova T, Kedrin D, Wyckoff J, Zhu L, Stanley ER et al (2009) The EGF/CSF-1 paracrine invasion loop can be triggered by heregulin \(\beta _{1}\) and CXCL12. Cancer Res 69(7):3221–3227
Knútsdóttir H, Pálsson E, Edelstein-Keshet L (2014) Mathematical model of macrophage-facilitated breast cancer cells invasion. J Theor Biol 357:184–199
Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56(20):4625–4629
Leung E, Xue A, Wang Y, Rougerie P, Sharma VP, Eddy R et al (2017) Blood vessel endothelium-directed tumor cell streaming in breast tumors requires the HGF/C-Met signaling pathway. Oncogene 36(19):2680
Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW (2002) Macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia 7:147–162
Lions JL (2013) Equations differentielles operationnelles: et problèmes aux limites, vol 111. Springer, Berlin
Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L (1992) The origin and function of tumor-associated macrophages. Immunol Today 13(7):265–270
Mantovani A, Schioppa T, Porta C, Allavena P, Sica A (2006) Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 25(3):315–322
Nagai T, Senba T (1997) Behavior of radially symmetric solutions of a system related to chemotaxis. Nonlinear Anal Theory Methods Appl 30(6):3837–3842
Perthame B (2006) Transport equations in biology. Springer, Berlin
Reymond N, d’Água BB, Ridley AJ (2013) Crossing the endothelial barrier during metastasis. Nat Rev Cancer 13(12):858
Roussos ET, Condeelis JS, Patsialou A (2011) Chemotaxis in cancer. Nat Rev Cancer 11(8):573
Simon J (1986) Compact sets in the space Lp (O, T; B). Ann Mat 146(1):65–96
Shafrir I, Wolansky G (2005) Moser–Trudinger and logarithmic HLS inequalities for systems. J Eur Math Soc 4:413–448
Shafrir I, Wolansky G (2005) The logarithmic HLS inequality for systems on compact manifolds. J Funct Anal 227(1):200–226
Shafrir I, Wolansky G (2005) Moser–Trudinger and logarithmic HLS inequalities for systems. J Eur Math Soc 7(4):413–448
Su S, Liu Q, Chen J, Chen J, Chen F, He C et al (2014) A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer cell 25(5):605–620
Tao Y, Winkler M (2015) Boundedness vs. blow-up in a two-species chemotaxis system with two chemicals. Discrete Contin Dyn Syst Ser B 20(9):3165–3183
Tao Y, Winkler M (2014) Energy-type estimates and global solvability in a two-dimensional chemotaxis-haptotaxis model with remodeling of non-diffusible attractant. J Differ Equ 257(3):784–815
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147(2):275–292
Wang W, Goswami S, Sahai E, Wyckoff JB, Segall JE, Condeelis JS (2005) Tumor cells caught in the act of invading: their strategy for enhanced cell motility. Trends Cell Biol 15(3):138–145
Wolansky G (1992) On steady distributions of self-attracting clusters under friction and fluctuations. Arch Ration Mech Anal 119(4):355–391
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER et al (2004) A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 64(19):7022–7029
Acknowledgements
The author gratefully acknowledges to Ph.D. Matthias Piesche, Facultad de Medicina, Universidad Católica del Maule, for his important comments for contextualize adequately in cancer progression the mathematical system studied in this work. The author gratefully acknowledges the comments on the first and the second drafts made by reviewers, which have greatly enriched this subsequent manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
For K. Vilches this work was funded by CONICYT PAI/ACADEMIA 79150021 2016–2018. For C. Conca this work is partially supported by PFBasal-001 and AFB170001 Projects.
Rights and permissions
About this article
Cite this article
Espejo, E., Vilches, K. & Conca, C. A simultaneous blow-up problem arising in tumor modeling. J. Math. Biol. 79, 1357–1399 (2019). https://doi.org/10.1007/s00285-019-01397-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-019-01397-6