Abstract
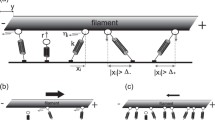
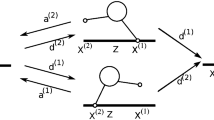
It is often assumed in biophysical studies that when multiple identical molecular motors interact with two parallel microtubules, the microtubules will be crosslinked and locked together. The aim of this study is to examine this assumption mathematically. We model the forces and movements generated by motors with a time-continuous Markov process and find that, counter-intuitively, a tug-of-war results from opposing actions of identical motors bound to different microtubules. The model shows that many motors bound to the same microtubule generate a great force applied to a smaller number of motors bound to another microtubule, which increases detachment rate for the motors in minority, stabilizing the directional sliding. However, stochastic effects cause occasional changes of the sliding direction, which has a profound effect on the character of the long-term microtubule motility, making it effectively diffusion-like. Here, we estimate the time between the rare events of switching direction and use them to estimate the effective diffusion coefficient for the microtubule pair. Our main result is that parallel microtubules interacting with multiple identical motors are not locked together, but rather slide bidirectionally. We find explicit formulae for the time between directional switching for various motor numbers.











Similar content being viewed by others
References
Aldous D, Fill JA (2002) Reversible Markov chains and random walks on graphs. http://www.stat.berkeley.edu/~aldous/RWG/book.html
Anderson W (1991) Continuous-time Markov chains: an applications-oriented approach. Applied probability. Springer. ISBN 9780387973692
Atzberger PJ, Peskin CS (2006) A Brownian dynamics model of kinesin in three dimensions incorporating the force-extension profile of the coiled-coil cargo tether. Bull Math Biol 68(1):131–160
Bell G (1978) Models for the specific adhesion of cells to cells. Science 200(4342):618–627
Bhat D, Gopalakrishnan M (2016) Transport of organelles by elastically coupled motor proteins. Eur Phys J E Soft Matter 39(7):71
Bouzat S (2016) Models for microtubule cargo transport coupling the Langevin equation to stochastic stepping motor dynamics: caring about fluctuations. Phys Rev E 93:012401
Craig EM, Yeung HT, Rao AN, Baas PW (2017) Polarity sorting of axonal microtubules: a computational study. Mol Biol Cell 28(23):3271–3285
del Castillo U, Winding M, Lu W, Gelfand VI (2015) Interplay between kinesin-1 and cortical dynein during axonal outgrowth and microtubule organization in Drosophila neurons. Elife 4:e10140
Eugene S, Xue W-F, Robert P, Doumic-Jauffret M (2016) Insights into the variability of nucleated amyloid polymerization by a minimalistic model of stochastic protein assembly. J Chem Phys 144(17):12
Fink G, Hajdo L, Skowronek KJ, Reuther C, Kasprzak AA, Diez S (2009) The mitotic kinesin-14 Ncd drives directional microtubule-microtubule sliding. Nat Cell Biol 11(6):717–723
Grimmett G, Grimmett P, Stirzaker D, Stirzaker M, Grimmett S (2001) Probability and random processes. OUP, Oxford. ISBN 9780198572220
Gross SP, Welte MA, Block SM, Wieschaus EF (2002) Coordination of opposite-polarity microtubule motors. J Cell Biol 156(4):715–724
Gross SP, Vershinin M, Shubeita GT (2007) Cargo transport: two motors are sometimes better than one. Curr Biol 17(12):R478–R486
Huisinga W, Meyn S, Schütte C (2004) Phase transitions and metastability in Markovian and molecular systems. ANNAP 14(1):419–458
Ikuta J, Kamisetty NK, Shintaku H, Kotera H, Kon T, Yokokawa R (2014) Tug-of-war of microtubule filaments at the boundary of a kinesin- and dynein-patterned surface. Sci Rep 4:5281
Klumpp S, Lipowsky R (2005) Cooperative cargo transport by several molecular motors. Proc Natl Acad Sci USA 102(48):17284–17289
Kunwar A, Tripathy SK, Xu J, Mattson MK, Anand P, Sigua R, Vershinin M, McKenney RJ, Yu CC, Mogilner A, Gross SP (2011) Mechanical stochastic tug-of-war models cannot explain bidirectional lipid-droplet transport. Proc Natl Acad Sci 108(47):18960–18965
Kural C, Kim H, Syed S, Goshima G, Gelfand VI, Selvin PR (2005) Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science 308(5727):1469–1472
Lee RH, Mitchell CS (2015) Axonal transport cargo motor count versus average transport velocity: is fast versus slow transport really single versus multiple motor transport? J Theor Biol 370:39–44
Levin DA, Peres Y, Wilmer EL (2009) Markov chains and mixing times. American Mathematical Society, Providence
Lu W, Gelfand VI (2017) Moonlighting motors: kinesin, dynein, and cell polarity. Trends Cell Biol 27(7):505–514
Lu W, Fox P, Lakonishok M, Davidson MW, Gelfand VI (2013) Initial neurite outgrowth in Drosophila neurons is driven by kinesin-powered microtubule sliding. Curr Biol 23(11):1018–1023
Ludecke A, Seidel AM, Braun M, Lansky Z, Diez S (2018) Diffusive tail anchorage determines velocity and force produced by kinesin-14 between crosslinked microtubules. Nat Commun 9(1):2214
McKinley SA, Athreya A, Fricks J, Kramer PR (2012) Asymptotic analysis of microtubule-based transport by multiple identical molecular motors. J Theor Biol 305:54–69
Miclo L (2015) An absorbing eigentime identity. Markov Proc Relat Fields 21(2):249–262
Miles CE, Keener JP (2017) Bidirectionality from cargo thermal fluctuations in motor-mediated transport. J Theor Biol 424:37–48
Müller MJI, Klumpp S, Lipowsky R (2008) Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc Natl Acad Sci 105(12):4609–4614
Nascimento AA, Roland JT, Gelfand VI (2003) Pigment cells: a model for the study of organelle transport. Annu Rev Cell Dev Biol 19:469–491
Newby JM, Bressloff PC (2010) Quasi-steady state reduction of molecular motor-based models of directed intermittent search. Bull Math Biol 72(7):1840–1866
Norris JR (1997) Markov chains. Cambridge series in statistical and probabilistic mathematics. Cambridge University Press, Cambridge
Oelz DB, Del Castillo U, Gelfand VI, Mogilner A (2018) Microtubule dynamics, kinesin-1 sliding, and dynein action drive growth of cell processes. Biophys J 115(8):1614–1624
Patel SR, Richardson JL, Schulze H, Kahle E, Galjart N, Drabek K, Shivdasani RA, Hartwig JH, Italiano JE (2005) Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes. Blood 106(13):4076–4085
Rogers SL, Gelfand VI (2000) Membrane trafficking, organelle transport, and the cytoskeleton. Curr Opin Cell Biol 12(1):57–62
Saito N, Kaneko K (2017) Embedding dual function into molecular motors through collective motion. Sci Rep 7:44288
Saloff-Coste L (1997) Lectures on finite Markov chains. In: Bernard P (ed) Lectures on probability theory and statistics. Lecture notes in mathematics, vol 1665. Springer, Berlin, pp 301–413
Sharp DJ, Brown HM, Kwon M, Rogers GC, Holland G, Scholey JM (2000) Functional coordination of three mitotic motors in Drosophila embryos. Mol Biol Cell 11(1):241–253
Shimamoto Y, Forth S, Kapoor TM (2015) Measuring pushing and braking forces generated by ensembles of kinesin-5 crosslinking two microtubules. Dev Cell 34(6):669–681
Svoboda K, Block SM (1994) Force and velocity measured for single kinesin molecules. Cell 77(5):773–784
Visscher K, Schnitzer MJ, Block SM (1999) Single kinesin molecules studied with a molecular force clamp. Nature 400(6740):184–189
Wollman R, Civelekoglu-Scholey G, Scholey JM, Mogilner A (2008) Reverse engineering of force integration during mitosis in the Drosophila embryo. Mol Syst Biol 4:195
Zhang Y, Fisher ME (2010) Dynamics of the tug-of-war model for cellular transport. Phys Rev E Stat Nonlinear Soft Matter Phys 82(1 Pt 1):011923
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study has been supported by NIH Grant R01 GM123068, NSF Grant DMS 1715455 and NSF Grant DMS 1763272 to JA; furthermore by ERC Starting Grant SKIPPERAD (number 306321) to MD, by ARC Discovery Project DP180102956 to DO and by NIH Grant GM121971 to AM.
Rights and permissions
About this article
Cite this article
Allard, J., Doumic, M., Mogilner, A. et al. Bidirectional sliding of two parallel microtubules generated by multiple identical motors. J. Math. Biol. 79, 571–594 (2019). https://doi.org/10.1007/s00285-019-01369-w
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-019-01369-w