Abstract
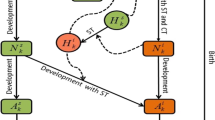
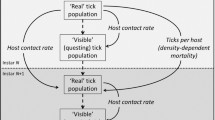
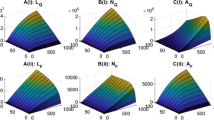
Ixodid ticks are acknowledged as one of the most important hematophagous arthropods because of their ability in transmitting a variety of tick-borne diseases. Mathematical models have been developed, based on emerging knowledge about tick ecology, pathogen epidemiology and their interface, to understand tick population dynamics and tick-borne diseases spread patterns. However, no serious effort has been made to model and assess the impact of host immunity triggered by tick feeding on the distribution of the tick population according to tick stages and on tick population extinction and persistence. Here, we construct a novel mathematical model taking into account the effect of host immunity status on tick population dynamics, and analyze the long-term behaviours of the model solutions. Two threshold values, \({\mathcal {R}}_{11}\) and \({\mathcal {R}}_{22}\), are introduced to measure the reproduction ratios for the tick-host interaction in the absence and presence of host immunity. We then show that these two thresholds (sometimes under additional conditions) can be used to predict whether the tick population goes extinct (\({\mathcal {R}}_{11}<1\)) and the tick population grows without bound (\({\mathcal {R}}_{22}>1\)). We also prove tick permanence (persistence and boundedness of the tick population) and the existence of a tick persistence equilibrium if \({\mathcal {R}}_{22}<1<{\mathcal {R}}_{11}\). As the host species adjust their immunity to tick infestation levels, they form for the tick population an environment with a carrying capacity very much like that in logistic growth. Numerical results show that the host immune reactions decrease the size of the tick population at equilibrium and apparently reduce the tick-borne infection risk.




Similar content being viewed by others
References
Awerbuch TE, Sandberg S (1995) Trends and oscillations in tick population dynamics. J Theor Biol 175:511–516
Barbalat I (1959) Systèmes d’équations différentielles d’oscillations nonlinéaires. Re Roum Math Pure Appl 4:267–270
Brossard M, Wikel SK (2004) Tick immunobiology. Parasitology 129(Suppl):S161–76
Brown SJ (1988) Highlights of contemporary research on host immune responses to ticks. Vet Parasitol 28(4):321–34
de la Fuente J, Villar M, Cabezas-Cruz A, Estrada-Peña A, Ayllón N, Alberdi P (2016) Tick-host-pathogen interactions: conflict and cooperation. PLoS Pathog 12(4):e1005488
Dipeolu OO, Mongi AO, Essuman S, Amoo AO, Ndungu JN (1992) Studies on naturally acquired immunity to African ticks. II. Observations on cattle exposed to Rhipicephalus appendiculatus under varying periods of repeated infestations. Vet Parasitol 41:293–320
Dizij A, Kurtenbach K (1995) Clethrionomys glareolus, but not Apodemus flavicollis, acquires resistance to Lxodes ricinus L., the main European vector of Borrelia burgdorferi. Parasite Immunol 17:177–183
Fan G, Thieme HR, Zhu H (2015a) Delay differential systems for tick population dynamics. J Math Biol 71:1017–48
Fan G, Lou Y, Thieme HR, Wu J (2015b) Stability and persistence in ODE models for populations with many stages. Math Biosci Eng 12:661–686
Gaff HD, Gross LJ (2007) Modeling tick-borne disease: a metapopulation model. Bull Math Biol 69:265–88
Gaff HD, Schaefer E (2010) Metapopulation models in tick-borne disease transmission modelling. Adv Exp Med Biol 673:51–65
Gilbert L, Norman R, Laurenson KM, Reid HW, Hudson PJ (2001) Disease persistence and apparent competition in a three-host community: an empirical and analytical study of large-scale, wild populations. J Anim Ecol 70:1053–1061
Hajdus̆ek O, Síma R, Ayllón N, Jalovecká M, Perner J, de la Fuente J, Kopác̆ek P (2013) Interaction of the tick immune system with transmitted pathogens. Front Cell Infect Microbiol 3:26
Hartemink NA, Randolph SE, Davis SA, Heesterbeek JAP (2008) The basic reproduction number for complex disease systems: defining \(R_0\) for tick-borne infections. Am Nat 171:743–754
Jones LD, Nuttall PA (1990) The effect of host resistance to tick infestation on the transmission of Thogoto virus by ticks. J Gen Virol 71:1039–43
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitology 129(Suppl):S3–14
Kaufman WR (2010) Ticks: physiological aspects with implications for pathogen transmission. Ticks Tick Borne Dis 1:11–22
Kovar L (2004) Tick saliva in anti-tick immunity and pathogen transmission. Folia Microbiol (Praha) 49:327–336
Lou Y, Liu L, Gao D (2017) Modeling co-infection of Ixodes tick-borne pathogens. Math Biosci Eng 14:1301–1316
Marsot M, Chapuis J-L, Gasqui P, Dozières A, Masséglia S, Pisanu B, Ferquel E, Vourch G (2013) Introduced Siberian chipmunks (Tamias sibiricus barberi) contribute more to Lyme Borreliosis risk than native reservoir rodents. PLoS ONE 8:55377. https://doi.org/10.1371/journal.pone.0055377
Matser A, Hartemink N, Heesterbeek H, Galvani A, Davis S (2009) Elasticity analysis in epidemiology: an application to tick-borne infections. Ecol Lett 12:1298–1305
Mount GA, Haile DG, Davey RB, Cooksey LM (1991) Computer simulation of boophilus cattle tick (Acari: Ixodidea) population dynamics. J Med Entomol 28:223–240
Mount GA, Haile DG, Daniels E (1997) Simulation of blacklegged tick (Acari: Ixodidea) population dynamics and transmission of borrelia burgdorferi. J Med Entomol 34:461–484
Nuttall PA, Paesen GC, Lawrie CH, Wang H (2000) Vector-host interactions in disease transmission. J Mol Microbiol Biotechnol 2:381–386
Ogden NH, Bigras-Poulin M, O’Callaghan CJ, Barker IK, Lindsay LR, Maarouf A, Smoyer-Tomic KE, Waltner-Toews D, Charron D (2005) A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int J Parasitol 35:375–389
Ogden NH, Radojevic M, Wu X, Duvvuri VR, Leighton PA, Wu J (2014) Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector Ixodes scapularis. Environ Health Perspect 122:631–8
Ostfeld RS, Keesing F (2000) Biodiversity and disease risk: the case of Lyme disease. Conserv Biol 14:722–728
Parola P, Raoult D (2001) Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis 32:897–928
Rosá R, Pugliese A (2007) Effects of tick population dynamics and host densities on the persistence of tick-borne infections. Math Biosci 208:216–240
Rosá R, Pugliese A, Norman R, Hudson PJ (2003) Thresholds for disease persistence in models for tick-borne infections including non-viraemic transmission, extended feeding and tick aggregation. J Theor Biol 224:359–376
Sandberg S, Awerbuch TE, Spielman A (1992) A comprehensive multiple matrix model representing the life cycle of the tick that transmits the age of Lyme disease. J Theor Biol 157:203–220
Smith HL (1995) Monotone dynamical systems: an introduction to the theory of competitive and cooperative systems. Amer Math Soc, Providence
Smith HL, Thieme HR (2011) Dynamical systems and population persistence. Amer Math Soc, Providence
Trager W (1939) Acquired immunity to ticks. J Parasitol 25:57–81
Thieme HR (2003) Mathematics in population biology. Princeton Univ Press, Princeton
Wang W, Zhao XQ (2015) Spatial invasion threshold of Lyme disease. SIAM J Appl Math 75:1142–1170
Wang X, Zhao XQ (2017) Dynamics of a time-delayed Lyme disease model with seasonality. SIAM J Appl Dyn Syst 16:853–881
Wikel SK (1996) Host immunity to ticks. Ann Rev Entomol 41:122
Wikel SK (1999) Tick modulation of host immunity: an important factor in pathogen transmission. Int J Parasitol 29:851–859
Wu X, Duvvuri VR, Lou Y, Ogden NH, Pelcat Y, Wu J (2013) Developing a temperature-driven map of the basic reproductive number of the emerging tick vector of Lyme disease Ixodes scapularis in Canada. J Theor Biol 319:50–61
Yu X, Zhao XQ (2016) A nonlocal spatial model for Lyme disease. J Differ Equ 261:340–372
Zhang Y, Zhao XQ (2013) A reaction-diffusion Lyme disease model with seasonality. SIAM J Appl Math 73:2077–2099
Zhao XQ (2003) Dynamical systems in population biology. Springer, New York
Acknowledgements
This project was initiated during the workshop on “Mathematics inspired by immunoepidemiology” held at the American Institute of Mathematics (August 24-28, 2015), and the authors and all rodents and deer plagued by ticks gratefully acknowledge the AIM’s support. This research was also financially supported by the National Natural Science Foundation of China (No.11501358, held by XW), the Natural Sciences and Engineering Research Council of Canada and the Canada Research Chairs program (JW), and by NSF grant DMS-1615879 (YK). The authors thank an anonymous referee for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jennings, R., Kuang, Y., Thieme, H.R. et al. How ticks keep ticking in the adversity of host immune reactions. J. Math. Biol. 78, 1331–1364 (2019). https://doi.org/10.1007/s00285-018-1311-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-018-1311-1
Keywords
- Host resistance
- Persistence
- Extinction
- Basic reproduction ratios
- Quasi-steady-state approximation
- Global stability