Abstract
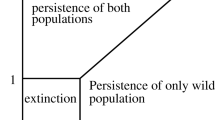
The control of the spread of dengue fever by introduction of the intracellular parasitic bacterium Wolbachia in populations of the vector Aedes aegypti, is presently one of the most promising tools for eliminating dengue, in the absence of an efficient vaccine. The success of this operation requires locally careful planning to determine the adequate number of individuals carrying the Wolbachia parasite that need to be introduced into the natural population. The introduced mosquitoes are expected to eventually replace the Wolbachia-free population and guarantee permanent protection against the transmission of dengue to human. In this study, we propose and analyze a model describing the fundamental aspects of the competition between mosquitoes carrying Wolbachia and mosquitoes free of the parasite. We then use feedback control techniques to devise an introduction protocol that is proved to guarantee that the population converges to a stable equilibrium where the totality of mosquitoes carry Wolbachia.

Similar content being viewed by others
Notes
See e.g. the page http://www.eliminatedengue.com/co/progress/article/690/ in the site of the project Eliminate Dengue (Hoffmann et al. 2012).
References
Alphey L (2014) Genetic control of mosquitoes. Annu Rev Entomol 59(1):205–224
Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, Dobson SL (2010) Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector-Borne Zoonotic Dis 10(3):295–311
Angeli D, Sontag ED (2003) Monotone control systems. IEEE Trans Autom Control 48:1684–1698
Angeli D, De Leenheer P, Sontag ED (2004) A small-gain theorem for almost global convergence of monotone systems. Syst Control Lett 52(5):407–414
Barton N, Turelli M (2011) Spatial waves of advance with bistable dynamics: cytoplasmic and genetic analogues of Allee effects. Am Nat 178(3):E48–E75
Bian G, Xu Y, Lu P, Xie Y, Xi Z (2010) The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6(4):e1000833
Bliman PA, Aronna MS, Coelho FC, da Silva MAHB (2015) Ensuring successful introduction of Wolbachia in natural populations of Aedes aegypti by means of feedback control. arxiv http://arxiv.org/abs/1503.05216
Brogdon WG, McAllister JC (1998) Insecticide resistance and vector control. Emerg Infect Dis 4(4):605–613
Brownstein JS, Hett E, O’Neill SL (2003) The potential of virulent Wolbachia to modulate disease transmission by insects. J Invertebr Pathol 84(1):24–29
Christophers S (1960) Aedes aegypti (L.) the yellow fever mosquito: its life history, bionomics and structure. Cambridge University Press, Cambridge
Coppel WA (1965) Stability and asymptotic behavior of differential equations. Heath, Heath mathematical monographs
de Freitas RM, Valle D (2014) Challenges encountered using standard vector control measures for dengue in Boa Vista. Brazil. Bull World Health Organ 92(9):685–689
de Freitas RM, Avendanho FC, Santos R, Sylvestre G, Araujo SC, Lima JBP, Martins AJ, Coelho GE, Valle D (2014) Undesirable consequences of insecticide resistance following Aedes aegypti control activities due to a dengue outbreak. PLoS ONE 9:1–9
Dutra HLC, Santos LMB, Caragata EP, Silva JBL, Villela DAM, Maciel-de Freitas R, Moreira LA (2015) From lab to field: the influence of urban landscapes on the invasive potential of Wolbachia in Brazilian Aedes aegypti mosquitoes. PLOS Negl Trop Dis 9(4):e0003689
Enciso G (2014) Fixed points and convergence in monotone systems under positive or negative feedback. Int J Control 87(2):301–311
Enciso G, Sontag ED (2006) Nonmonotone systems decomposable into monotone systems with negative feedback. J Differ Equ 224:205–227
Farnesi LC, Martins AJ, Valle D, Rezende GL (2009) Embryonic development of Aedes aegypti (Diptera: Culicidae): influence of different constant temperatures. Mem Inst Oswaldo Cruz 104(1):124–6
Ferreira CP, Godoy WA (eds) (2014) Ecological modelling applied to entomology. Springer, Berlin
Focks DA (2003) A review of entomological sampling methods and indicators for dengue vectors. WHO, Geneva
Focks DA, Haile DG, Daniels E, Mount GA (1993) Dynamic life table model of Aedes aegypti (Diptera: Culicidae)—analysis of the literature and model development. J Med Entomol 30:1003–1017
Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, McGraw EA, O’Neill SL (2014) Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis 8(2):e2688
Gedeon T, Hines G (2009) Multi-valued characteristics and morse decompositions. J Differ Equ 247(4):1013–1042
Gouzé JL (1988) A criterion of global convergence to equilibrium for differential systems with an application to Lotka–Volterra systems. Research report 0894, Inria, France
Hancock PA, Godfray HCJ (2012) Modelling the spread of Wolbachia in spatially heterogeneous environments. J R Soc Interface 9(76):3045–3054
Hancock PA, Sinkins SP, Godfray HCJ (2011a) Population dynamic models of the spread of Wolbachia. Am Nat 177(3):323–333
Hancock PA, Sinkins SP, Godfray HCJ (2011b) Strategies for introducing Wolbachia to reduce transmission of mosquito-borne diseases. PLoS Negl Trop Dis 5(4):e1024
Hirsch MW (1988) Stability and convergence in strongly monotone dynamical systems. J Reine Angew Math 383:1–53
Hoffmann AA (2014) Facilitating Wolbachia invasions. Aust Entomol 53(2):125–132
Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, Ritchie SA, Turelli M, O’Neill SL (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476(7361):454–457
Hoffmann A, Moreira L, O’Neill S, Osorio JE, Ritchie S, Simmons C, Turelli M (2012), Eliminate dengue. http://www.eliminatedengue.com/
Hu L, Huang M, Tang M, Yu J, Zheng B (2015) Wolbachia spread dynamics in stochastic environments. Theor Popul Biol 106:32–44
Huang M, Tang M, Yu J (2015) Wolbachia infection dynamics by reaction–diffusion equations. Sci China Math 58(1):77–96
Hughes H, Britton NF (2013) Modelling the use of Wolbachia to control dengue fever transmission. Bull Math Biol 75(5):796–818
Jeffery JAL, Yen NT, Nam VS, Nghia LT, Hoffmann AA, Kay BH, Ryan PA (2009) Characterizing the Aedes aegypti population in a Vietnamese village in preparation for a Wolbachia-based mosquito control strategy to eliminate dengue. PLOS Negl Trop Dis 3(11):e552
Keeling MJ, Jiggins FM, Read JM (2003) The invasion and coexistence of competing Wolbachia strains. Heredity 91(4):382–388
Koiller J, Da Silva M, Souza M, Codeço C, Iggidr A, Sallet G (2014) Aedes, Wolbachia and Dengue. Research report RR-8462, Inria, France
La Salle JP (1976) The stability of dynamical systems. SIAM, Philadelphia
Malisoff M, De Leenheer P (2006) A small-gain theorem for monotone systems with multi-valued input-state characteristics. IEEE Trans Autom Control 41(2):287–292
McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, Wang YF, O’Neill SL (2009) Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323(5910):141–4
Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O’Neill SL (2009a) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell 139(7):1268–1278
Moreira LA, Saig E, Turley AP, Ribeiro JMC, O’Neill SL, McGraw AE (2009) Human probing behavior of Aedes aegypti when infected with a life-shortening strain of Wolbachia. PLOS Negl Trop Dis 12(3):1–6
Murray JV, Jansen CC, De Barro P (2016) Risk associated with the release of Wolbachia-infected Aedes aegypti mosquitoes into the environment in an effort to control dengue. Front Public Health 4:43
Ndii MZ, Hickson R, Allingham D, Mercer G (2015) Modelling the transmission dynamics of dengue in the presence of Wolbachia. Math Biosci 262:157–166
O’Neill S (2015) The dengue stopper. Sci Am 312(6):72–7
O’Neill SL, Hoffman AA, Werren JH (eds) (1998) Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford
Ocampo CB, Salazar-Terreros MJ, Mina NJ, McAllister J, Brogdon W (2011) Insecticide resistance status of Aedes aegypti in 10 localities in Colombia. Acta Trop 118(1):37–44
Otero M, Solari HG, Schweigmann N (2006) A stochastic population dynamics model for Aedes aegypti: formulation and application to a city with temperate climate. Bull Math Biol 68:1945–1974
Otero M, Schweigmann N, Solari HG (2008) A stochastic spatial dynamical model for Aedes aegypti. Bull Math Biol 70:1297–1325
Rasgon JL, Scott TW (2004) An initial survey for Wolbachia (Rickettsiales: Rickettsiaceae) infections in selected California mosquitoes (Diptera: Culicidae). J Med Entomol 41(2):255–257
Ruang-Areerate T, Kittayapong P (2006) Wolbachia transinfection in Aedes aegypti: a potential gene driver of dengue vectors. Proc Natl Acad Sci 103(33):12534–9
Silver JB (2007) Mosquito ecology: field sampling methods. Springer, Berlin
Smith HL (1995) Monotone dynamical systems: an introduction to the theory of competitive and cooperative systems. Mathematical surveys and monographs, vol 41. American Mathematical Society
Smith DL, Perkins TA, Tusting LS, Scott TW, Lindsay SW (2013) Mosquito population regulation and larval source management in heterogeneous environments. PLoS One 8(8):e71247
Southwood TR, Murdie G, Yasuno M, Tonn RJ, Reader PM (1972) Studies on the life budget of Aedes aegypti in Wat Samphaya, Bangkok, Thailand. Bull World Health Organ 46(2):211
Turelli M (2010) Cytoplasmic incompatibility in populations with overlapping generations. Evolution 64(1):232–241
Walker T, Johnson P, Moreira L, Iturbe-Ormaetxe I, Frentiu F, McMeniman C, Leong YS, Dong Y, Axford J, Kriesner P et al (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476(7361):450–453
Xi Z, Khoo CC, Dobson SL (2005) Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310(5746):326–8
Yang HM, Macoris ML, Galvani KC, Andrighetti MT, Wanderley DM (2009) Assessing the effects of temperature on the population of Aedes aegypti, the vector of dengue. Epidemiol Infect 137(08):1188–1202
Yeap HL, Mee P, Walker T, Weeks AR, O’Neill SL, Johnson P, Ritchie SA, Richardson KM, Doig C, Endersby NM, Hoffmann AA (2011) Dynamics of the popcorn Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetic 187(2):583–95
Zheng B, Tang M, Yu J (2014) Modeling Wolbachia spread in mosquitoes through delay differential equations. SIAM J Appl Math 74(3):743–770
Acknowledgements
We wish to thank the anonymous referees for their valuable advices that helped improve this manuscript. The first author is indebted to T. Gedeon for valuable discussions. The second author thanks H. Solari for useful bibliographical references. This work was done while the second author was a postdoctoral fellow at IMPA, Rio de Janerio, funded by CAPES-Brazil. The authors also acknowledge CAPES-Brazil for the STIC AmSud funding for the MOSTICAW Project, Process No. 99999.007551/2015-00, and FGV for the Pesquisa Aplicada funding of the project “Controle da Dengue através do uso da bactéria Wolbachia”.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A: A sexual version of the infestation model
We here provide a sexual version of model (1). For simplicity, no control input is written. We denote respectively \({\mathbf {m}}_U, {\mathbf {m}}_W, {\mathbf {M}}_U, {\mathbf {M}}_W\), the numbers of uninfected, resp. Wolbachia-infected, males in early and adult phases; and similarly \({\mathbf {f}}_U, {\mathbf {f}}_W, {\mathbf {F}}_U, {\mathbf {F}}_W\) for the females. The model is:
Here \(\lambda _U, \lambda _W\) are the sex ratio (ratio of males to females) of the offspring for the uninfected and infected populations. The other parameters have the same meaning than for model (1) (see Table 2). Here they have been chosen identical for both sex, and in such conditions, it is straightforward to see that the variables defined by
obey the following equations:
If moreover \(\lambda _U=\lambda _W\) and the sex ratio are initially equal to this common value, that is:
then the same proportions are conserved along the evolution, and it is possible to replace \(\frac{{\mathbf {M}}_U}{{\mathbf {M}}_U+{\mathbf {M}}_W}\) by \(\frac{{\mathbf {L}}_U}{{\mathbf {L}}_U+{\mathbf {L}}_W}, (1+\lambda _U) {\mathbf {F}}_U\) by \({\mathbf {L}}_U\) and \((1+\lambda _W) {\mathbf {F}}_W\) by \({\mathbf {L}}_W\) in (41a), (41c), showing that (41) boils down to the simpler model (1).
Appendix B: Proof of Theorem 7
1.1 Computation and ordering of the equilibrium points
One here computes the equilibrium points. The latter verify
The point \(x_{0,0} := (0,0,0,0)\) is clearly an equilibrium. Let us look for an equilibrium \(x_{U,0} := (L_U^*,A_U^*,0,0)\). The quantities \(L_U^*,A_U^*\) then have to satisfy
Dividing by \(L_U^*\ne 0\) yields \(1+ L_U^* = \mathcal{R}_U\). One thus gets the unique solution of this form verifying
which is positive due to the sustainability hypothesis (6).
Similarly, one now looks for an equilibrium defined as \(x_{0,W} := (0,0,L_W^*,A_W^*)\). The values of \(L_W^*,A_W^*\) must verify
This is identical to (44), and as for the \(x_{U,0}\) case, one gets a unique, positive, solution, namely
We show now that system (7) also admits a unique coexistence equilibrium with positive components \(x_{U,W}= (L_U^{**}, A_U^{**}, L_W^{**}, A_W^{**})\). Coming back to (45) and expressing the value of the factor common to the first and second identity leads to
One thus deduces
and one can express all three remaining unknowns in function of \(A_W^{**}\):
Using the value of \(L_U^{**}\) and \(L_W^{**}\) now yields the relation
which has a unique, positive, solution when (6) holds. Hence, the fourth equilibrium is given by
where \(\delta \) was given in Eq. (14d) in the statement of the theorem.
So far we have found all equilibrium points. Actually, it is easy to see that no equilibria is missing: for \(L_U= 0\) we necessarily have \(A_U=0,\) and this gives us \(x_{0,0}\) and \(x_{0,W},\) while for \(L_U \ne 0 \) we get \(A_U\ne 0,\) and this leads us to \(x_{U,0}\) and to \(x_{U,W}.\)
Notice that the last equilibrium can be expressed alternatively by use of the values of the equilibrium \(x_{0,W}\):
and this provides straightforward comparison result:
and thus \(A_\eta ^{**} = \gamma _\eta L_\eta ^{**} < \gamma _\eta L_\eta ^* = A_\eta ^*\), for \(\eta \in \{U,W\}\) and, therefore,
the second inequality being directly deduced from (46b). The relations (50) allow us to establish the inequalities (15).
1.2 Local stability analysis
The local stability analysis is conducted through analysis of the eigenvalues of the Jacobian matrices. Recall that the Jacobian has been computed in (13).
Stability of \(x_{0,0}\). The value of \({ Df}\) is not defined at \(x_{0,0}\). To show the instability of the equilibrium \(x_{0,0}\), let the function V be defined on \(\mathbb {R}_+^4\) by
for values of \(\varepsilon , \rho \) still to be defined. Notice that V is positive definite for any positive \(\varepsilon \) and \(\rho .\) We will show that there exists \(\rho >0\) for which the derivative \({\dot{V}}\) of V along the trajectories is positive definite in a sufficiently small relative neighborhood of \(x_{0,0}\) in \(\mathbb {R}_+^4.\)
One can check that
The first term of the last expression is positive for all values of \((L_U,L_W)\) in some relative neighborhood of the origin in \(\mathbb {R}_+^2\). Assuming from now on that \(\varepsilon \in (0,\mathcal{R}_W-1)\), one verifies easily that the sum of the two remaining terms in the right-hand side of (48) is positive when exactly one of the two numbers \(A_U, A_W\) is zero, due to (6). Assume now that e.g. \(A_U\ne 0\). Then the sum of the last two terms in (48) is equal to
We will now prove that there exists \(b>0\) (and therefore \(\rho >0\)) such that the previous expression is positive for any nonnegative a (and therefore for any pair \((A_U,A_W)\) with \(A_U>0, A_W\ge 0\)).
The map
is clearly convex. It has a positive value at the origin, where its derivative is equal to \(-b\mathcal{R}_U+\mathcal{R}_W-1-\varepsilon \). Taking now \(0<b<\frac{\mathcal{R}_W-1-\varepsilon }{\mathcal{R}_U}\), this expression is positive, which ensures that the map (49) takes on positive values on \(\mathbb {R}^+\). Set \(\rho :=\frac{b\gamma _W}{\gamma _U}.\)
Therefore, positive values of \(\varepsilon \) and \(\rho \) have been exhibited, for which \({\dot{V}}\) is positive definite in a relative neighborhood of \(x_{0,0}\) in \(\mathbb {R}_+^4.\) This demonstrates the instability of \(x_{0.0}.\)
Stability of \(x_{U,0}\). Using (13) and recalling the value of \(x_{U,0}\) given in (16), we see that \({ Df}(x_{U,0})\) is the upper block-triangular matrix
The eigenvalues of this block-triangular matrix have negative real parts if and only if
These conditions are satisfied since the sustainability condition (6) holds. In conclusion, the equilibrium \(x_{U,0}\) is locally asymptotically stable.
Stability of \(x_{0,W}\). From (13) and (16) we get that the Jacobian \({ Df}(x_{0,W})\) of f at \(x_{0,W}\) is the lower block-triangular matrix
The left-upper \(2\times 2\)-block is a Hurwitz matrix, while asymptotic stability of the second one is equivalent to the condition
that is \(\mathcal{R}_W>1\), which holds true, due to hypothesis (6). The equilibrium \(x_{0,W}\) is thus locally asymptotically stable.
Stability of \(x_{U,W}\). The instability of \(x_{U,W}\) can be proved by showing that the determinant of the Jacobian matrix \({ Df}(x_{U,W})\) is negative, which, together with the fact that the state space has even dimension 4, establishes the existence of a positive real root to the characteristic polynomial; and thus that the Jacobian is not a Hurwitz matrix. This argument yields lengthy computations.
It is more appropriate to use here the monotonicity properties of system (7), established in Theorem 5. As a matter of fact, bringing together the inequalities (15) (already proved in the end of the previous section, see (50)), the asymptotical stability of \(x_{U,0}\) and \(x_{0,W}\) and the strongly order-preserving property of the reference problem, Theorem 2.2 in Smith (1995) shows that the intermediate point \(x_{U,W}\) cannot be stable. This finally achieves the stability analysis, as well as the proof of Theorem 7.
Rights and permissions
About this article
Cite this article
Bliman, PA., Aronna, M.S., Coelho, F.C. et al. Ensuring successful introduction of Wolbachia in natural populations of Aedes aegypti by means of feedback control. J. Math. Biol. 76, 1269–1300 (2018). https://doi.org/10.1007/s00285-017-1174-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-017-1174-x