Abstract
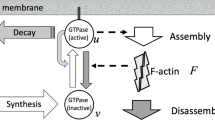
The processes of protrusion and retraction during cell movement are driven by the turnover and reorganization of the actin cytoskeleton. Within a reaction–diffusion model which combines processes along the cell membrane with processes within the cytoplasm a Turing type instability is used to form the necessary polarity to distinguish between cell front and rear and to initiate the formation of different organizational arrays within the cytoplasm leading to protrusion and retraction. A simplified biochemical network model for the activation of GTPase which accounts for the different dimensionality of the cell membrane and the cytoplasm is used for this purpose and combined with a classical Helfrich type model to account for bending and stiffness effects of the cell membrane. In addition streaming within the cytoplasm and the extracellular matrix is taken into account. Combining these phenomena allows to simulate the dynamics of cells and to reproduce the primary phenomenology of cell motility. The coupled model is formulated within a phase field approach and solved using adaptive finite elements.










Similar content being viewed by others
References
Allain J, Ben Amar M (2004) Biphasic vesicle: instability induced by adsorption of proteins. Phys A 337:531–545
Altschuler SJ, Angenent SB, Wang Y, Wu LF (2008) On the spontaneous emergence of cell polarity. Nature 454:886–889
Barnhart E, Lee KC, Keren K, Mogilner A, Theriot J (2011) An adhesion-dependent switch between mechanisms that determine mitile cell shape. PLOS Biol 9:e1001,059
Biben T, Kassner K, Misbah C (2005) Phase-field approach to three-dimensional vesicle dynamics. Phys Rev E 72:1–15
Bonito A, Nochetto R, Pauletti M (2011) Dynamics of biomembranes: effect of the bulk fluid. Math Model Nat Phenom 6:25–43
Das S, Du Q (2008) Adhesion of vesicles to curved substrates. Phys Rev E 77
Du Q, Li M, Liu C (2007) Analysis of a phase field Navier–Stokes vesicle–fluid interaction model. Discrete Contin Dyn Syst Ser B 8:539–556
Du Q, Liu C, Ryham R, Wang X (2005) A phase field formulation of the Willmore problem. Nonlinearity 18:1249
Du Q, Liu C, Ryham R, Wang X (2005) Modeling the spontaneous curvature effects in static cell membrane deformations by a phase field formulation. Commun Pure Appl Anal 4:537–548
Du Q, Liu C, Ryham R, Wang X (2009) Energetic variational approaches in modeling vesicle and fluid interactions. Phys D 238:923–930
Du Q, Liu C, Wang X (2006) Simulating the deformation of vesicle membranes under elastic bending energy in three dimensions. J Comput Phys 212:757–777
Du Q, Zhang J (2008) Adaptive finite element method for a phase field bending elasticity model of vesicle membrane deformations. SIAM J Sci Comput 30:1634–1657
Elliott C, Stinner B (2010) A surface phase field model for two-phase biological membranes. SIAM J Appl Math 70:2904–2928
Elliott CM, Stinner B, Venkataraman C (2012) Modelling cell motility and chemotaxis with evolving surface finite elements. J R Soc Interface 9:3027-3044
Enculescu M, Gholami A, Falcke M (2008) Dynamic regimes and bifurcations in a model of actin-based motility. Phys Rev E 78:031,915
Enculescu M, Sabouri-Ghorni M, Danuser G, Falcke M (2010) Modeling of protrution phenotypes driven by the actin–membrane interaction. Biophys J 98:1571–1581
Evans E, Rawicz W (1990) Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys Rev Lett 64:2094–2097
Goryachev AB, Pokhilko AV (2008) Dynamics of Cdc42 network embodies a Turing-type mechanism of yeast cell polarity. FEBS Lett 582:1437–1443
Haußer F, Li S, Lowengrub J, Marth W, Rätz A, Voigt A (2013) Thermodynamically consistent models for two-component vesicles (submitted)
Helfrich W (1973) Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch 28:693–703
Jilkine A, Edelstein-Keshet L (2011) A comparison of mathematical models for polarization of single eukaryotic cells in response to guided cues. PLoS Comput Biol 7:e1001121
Landsberg C, Stenger F, Deutsch A, Gelinsky M, Roesen-Wolff A, Voigt A (2011) Chemotaxis of mesenchymal stem cells within 3D biomimetic scaffolds—a modeling approach. J Biomech 44: 359–364
Levine H, Rappel WJ (2005) Membrane-bound turing patterns. Phys Rev E 72:061,912
Li X, Lowengrub J, Raetz A, Voigt A (2009) Solving pde’s in complex geometries: a diffuse domain approach. Commun Math Sci 7:81–107
Lowengrub J, Xu J, Voigt A (2007) Surface phase separation and flow in a simple model of multicomponent drops and vesicles. Fluid Dyn Math Proc 3:13–28
Lowengrub JS, Rätz A, Voigt A (2009) Phase-field modeling of the dynamics of multicomponent vesicles: spinodal decomposition, coarsening, budding, and fission. Phys Rev E 79:031,926
Maree AFM, Jilkine A, Dawes A, Grieneisen VA, Edelstein-Keshet L (2006) Polarization and movement of keratocytes: a multiscale modelling approach. Bull Math Biol 68:1169–1211
McMahon H, Gallop J (2005) Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438:590–596
Rätz A, Röger M (2012) Turing instabilities in a mathematical model for signaling networks. J Math Biol 65:1215–1244
Rätz A, Röger M (2013) Symmetry breaking in a bulk-surface reaction-diffusion model for signaling networks (preprint)
Rätz A, Voigt A (2006) Pde’s on surfaces—a diffuse interface approach. Commun Math Sci 4:575–590
Ryham R, Cohen FS, Eisenberg R (2012) A dynamic model of open vesicles in fluids. Commun Math Sci 10:1273–1285
Salac D, Miksis M (2011) A level set projection model of lipid vesicles in general flows. J Comput Phys 230:8192–8215
Seifert U (1997) Configurations of fluid membranes and vesicles. Adv Phys 46:13–137
Seifert U, Lipowsky R (1990) Adhesion of vesicles. Phys Rev A 42:4768–4771
Shao D, Levine H, Rappel WJ (2012) Coupling actin flow, adhesion, and morphology in a computational cell motility model. PNAS 109:6851–6856
Shao D, Rappel WJ, Levine H (2010) Computational model for cell morphodynamics. Phys Rev Lett 105:108,104
Simson R, Wallraff E, Faix J, Niewohner J, Gerisch G, Sackmann E (1998) Membrane bending modulus and adhesion energy of wild-type and mutant cells of Dictyostelium lacking talin or cortexillins. Biophys J 74:514–522
Sohn JS, Tseng YH, Li S, Voigt A, Lowengrub JS (2010) Dynamics of multicomponent vesicles in a viscous fluid. J Comput Phys 229:119–144
Strey H, Peterson M (1995) Measurment of erythrocyte-membrane elasticity by flicker eigenmode decomposition. Biophys J 69:478–488
Teigen KE, Li X, Lowengrub J, Wang F, Voigt A (2009) A diffuse-interface approach for modeling transport, diffusion and adsorption/desorption of material quantities on a deformable interface. Commun Math Sci 7:1009–1037
Teigen KE, Song P, Lowengrub J, Voigt A (2011) A diffuse-interface method for two-phase flows with soluble surfactants. J Comput Phys 230(2):375–393
Vanderlei B, Feng JJ, Edelstein-Keshet L (2011) A computational model of cell polarization and motility coupling mechanics and biochemistry. Multiscale Model Simul 9:1420–1443
Veksler A, Gov NS (2007) Phase transitions of the coupled membrane-cytoskeleton modify cellular shape. Biophys J 93:3798–3810
Vey S, Voigt A (2007) Amdis: adaptive multidimensional simulations. Comput Visual Sci 10:57–67
Voigt A, Witkowski T (2012) A multi-mesh finite element method for lagrange elements of arbitrary degree. J Comput Sci 3:420–428
Wang X, Du Q (2008) Modelling and simulations of multi-component lipid membranes and open membranes via diffuse interface approaches. J Math Biol 56:347–371
Wedlich-Soldner R, Altschuler S, Wu L, Li R (2003) Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299:1231–1235
Wedlich-Soldner R, Wai S, Schmidt T, Li R (2004) Robust cell polarity is a dynamic state established by coupling transport and GTPase SIGNALING. J Cell Biol 166:889–900
Zhelev DV, Needham D, Hochmuth R (1994) A novel micropipet method for measuring the bending modulus for vesicle membranes. Biophys J 67:720–727
Ziebert F, Swaminathan S, Aranson IS (2012) Model for self-polarization and motility of keratocyte fragments. J Roy Soc Interface 9:1084–1092
Acknowledgments
The work has been funded through grant DFG Vo899/6 and FP7 IRSES 247504. We further acknowledge the provided computing resources at ZIH at TU Dresden.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marth, W., Voigt, A. Signaling networks and cell motility: a computational approach using a phase field description. J. Math. Biol. 69, 91–112 (2014). https://doi.org/10.1007/s00285-013-0704-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-013-0704-4