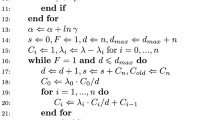Abstract
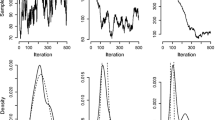
CFSE based tracking of the lymphocyte proliferation using flow cytometry is a powerful experimental technique in immunology allowing for the tracing of labelled cell populations over time in terms of the number of divisions cells undergone. Interpretation and understanding of such population data can be greatly improved through the use of mathematical modelling. We apply a heterogenous linear compartmental model, described by a system of ordinary differential equations similar to those proposed by Kendall. This model allows division number-dependent rates of cell proliferation and death and describes the rate of changes in the numbers of cells having undergone j divisions. The experimental data set that we specifically analyze specifies the following characteristics of the kinetics of PHA-induced human T lymphocyte proliferation assay in vitroL (1) the total number of live cells, (2) the total number of dead but not disintegrated cells and (3) the number of cells divided j times. Following the maximum likelihood approach for data fitting, we estimate the model parameters which, in particular, present the CTL birth- and death rate “functions”. It is the first study of CFSE labelling data which convincingly shows that the lymphocyte proliferation and death both in vitro and in vivo are division number dependent. For the first time, the confidence in the estimated parameter values is analyzed by comparing three major methods: the technique based on the variance–covariance matrix, the profile-likelihood-based approach and the bootstrap technique. We compare results and performance of these methods with respect to their robustness and computational cost. We show that for evaluating mathematical models of differing complexity the information-theoretic approach, based upon indicators measuring the information loss for a particular model (Kullback–Leibler information), provides a consistent basis. We specifically discuss methodological and computational difficulties in parameter identification with CFSE data, e.g. the loss of confidence in the parameter estimates starting around the sixth division. Overall, our study suggests that the heterogeneity inherent in cell kinetics should be explicitly incorporated into the structure of mathematical models.
Similar content being viewed by others
References
Abu-Absi N.R., Zamamiri A., Kacmar J., Balogh S.J., Srienc F. (2003) Automated flow cytometry for acquisition of time-dependent population data. Cytometry A 51, 87–96
Baker C.T.H., Bocharov G.A., Ford J.M., Lumb P.M., Norton S.J., Paul C.A.H., Junt T., Ludewig B., Krebs P. (2005) Computational approaches to parameter estimation and model selection in immunology. J. Comput. Appl. Math. 184, 50–76
Baker C.T.H., Bocharov G.A., Paul C.A.H., Rihan F.A. (2005) Computational modelling with functional differential equations: identification, selection and sensitivity. Appl. Numer. Math. 53, 107–129
Bard Y. (1974) Nonlinear Parameter Estimation. Academic, New York
Bernard S., Pujo-Menjouet L., Mackey M.C. (2003) Analysis of cell kinetics using a cell division marker: mathematical modeling of experimental data. Biophys. J. 84, 3414–3424
Burnham K.P., Anderson D.R., (2002) Model selection and multimodel inference—a practical information-theoretic approach, 2nd ed. Springer, Berlin Heidelberg New York
De Boer R.J., Perelson A.S. (2005) Estimating division and death rates from CFSE data. J. Comput. Appl. Math. 184(1): 140–164
Efron B., Tibshirani R. (1986) Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci. 1(1): 54–77
Efron B., Tibshirani R. (1993) Introduction to the bootstrap. Chapman and Hall, New York
Eisen M. (1979) Mathematical models in cell biology and cancer chemotherapy. Springer, Berlin Heidelberg New York
Ganusov V.V., Pilyugin S.S., De Boer R.J., Murali-Krishna K., Ahmed R., Antia R. (2005) Quantifying cell turnover using CFSE data. J. Immunol. Methods 298, 183–200
Gett A.V., Hodgkin P.D. (2000) A cellular calculus for signal integration by T cells. Nat. Immunol. 1, 239–244
Gudmundsdottir H., Wells A.D., Turka L.A. (1999) Dynamics and requirements of T cell clonal expansion in vivo at the single-cell level: effector function is linked to proliferative capacity. J. Immunol. 162: 5212–5223
Kendall D.G. (1948) On the role of variable generation time in the development of a stochastic birth process. Biometrika 35, 316–330
Murali-Krishna K., Ahmed R. (2000) Cutting edge: naive T cells masquerading as memory cells. J. Immunol. 165: 1733–1737
Muyng I.J., Balasubramanian V., Pitt M.A. (2000) Counting probability distributions: differential geometry and model selection. PNAS USA 97: 11170–11175
Pilyugin S.S., Ganusov V.V., Murali-Krishna K., Ahmed R., Antia R. (2003) The rescaling method for quantifying the turnover of cell populations. J. Theoret. Biol. 225(2): 275–283
Rubinov S.I. (1980) Cell kinetics. In: Segel L.A. (eds) Mathematical Models in Molecular and Cellular Biology. Cambridge University Press, Cambridge, pp 502–522
Smith J.A., Martin L.(1973) Do cell cycle? Proc Nat. Acad. Sci. USA 70(4): 1263–1267
Veiga-Fernandes H., Walter U., Bourgeois C., McLean A., Rocha B. (2000) Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat. Immunol. 1, 47–53
Venzon D.J., Moolgavkar S.H. (1988) A method for computing profile-likelihood-based confidence intervals. Appl. Statist. 37(1): 87–94
Wells A.D., Gudmundsdottir H., Turka L.A. (1997) Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J. Clin. Invest. 100, 3173–3183
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luzyanina, T., Mrusek, S., Edwards, J.T. et al. Computational analysis of CFSE proliferation assay. J. Math. Biol. 54, 57–89 (2007). https://doi.org/10.1007/s00285-006-0046-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-006-0046-6
Keywords
- Modelling T-cell kinetics
- CFSE assay
- Heterogenous compartmental model
- Parameter estimation
- Computation of confidence intervals