Abstract
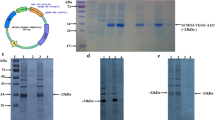
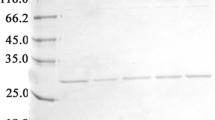
The DNA sequence coding for plasminogen kringle 5 (pK5), an inhibitor of angiogenesis, was fused with that coding for interferon gamma and over-produced in the form of inactive inclusion bodies in E. coli. The amount of fusion protein was about 40% of total protein produced. The fusion protein contained in the inclusion bodies was solubilized in 8 m urea and purified by anion-exchange chromatography. We employed the orthogonal experimental design L16(45) (5 factors, 4 levels, 16 experiments) procedure for researching the influence of denaturant, aggregation suppressor l-arginine, NaCl, pH, and glycine on the refolding procedure. Our results suggest that the presence of appropriate l-arginine, NaCl, and denaturant in the refolding buffer inhibits the aggregation of the fusion protein and increases the yield of renatured protein with biological activity. The refolded fusion protein, γIFN/pk5, has in vitro anti-endothelial cell proliferation activity.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 24 July 2000 / Accepted: 21 September 2000
Rights and permissions
About this article
Cite this article
Lu, H., Zhang, H., Wang, Q. et al. Purification, Refolding of Hybrid hIFNγ-Kringle 5 Expressed in Escherichia coli . Curr Microbiol 42, 211–216 (2001). https://doi.org/10.1007/s002840010206
Issue Date:
DOI: https://doi.org/10.1007/s002840010206