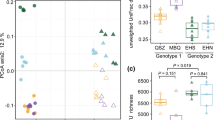
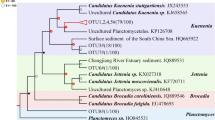
Abstract
Rhizospheric microbial community of emergent macrophytes plays an important role in nitrogen removal, especially in the eutrophic wetlands. The objective of this study was to identify the differences in anammox bacterial community composition among different emergent macrophytes and investigate revealed the the main factors affecting on the composition, diversity, and abundance of anammox bacterial community. Results showed that the composition, diversity, and abundance of the anammox community were significantly different between the vegetated sediments of three emergent macrophytes and unvegetated sediment. The composition of the anammox bacterial community was different in the vegetated sediments of different emergent macrophytes. Also, the abundance of nitrogen cycle-related functional genes in the vegetated sediments was found to be higher than that in the unvegetated sediment. Canonical correspondence analysis (CCA) and structural equation models analysis (SEM) showed that salinity and pH were the main environmental factors influencing the composition and diversity of the anammox bacterial community and NO2−-N indirectly affected anammox bacterial community diversity by affecting TOC. nirK-type denitrifying bacteria abundance had significant effects on the bacterial community composition, diversity, and abundance of anammox bacteria. The community composition of anammox bacteria varies with emergent macrophyte species. The rhizosphere of emergent macrophytes provides a favorable environment and promotes the growth of nitrogen cycling-related microorganisms that likely accelerate nitrogen removal in eutrophic wetlands.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
References
Bhagowati B, Ahamad KU (2018) A review on lake eutrophication dynamics and recent developments in lake modeling. Ecohydrol Hydrobiol 19(1):155–166. https://doi.org/10.1016/j.ecohyd.2018.03.002
Mulder A, VandeGraaf AA, Robertson LA, Kuenen JG (1995) Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiol Ecol 16:177–184. https://doi.org/10.1016/0168-6496(94)00081-7
Thamdrup B, Dalsgaard T (2002) Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl Environ Microbiol 68:1312–1318. https://doi.org/10.1128/AEM.68.3.1312-1318.2002
Nie S, Li H, Yang XY, Zhang ZJ, Weng B, Huang FY, Zhu GB, Zhu YG (2015) Nitrogen loss by anaerobic oxidation of ammonium in rice rhizosphere. ISME J. https://doi.org/10.1038/ismej.2015.25
Hua YM, Peng L, Zhang SH, Heal KV, Zhao JW, Zhu DW (2017) Effects of plants and temperature on nitrogen removal and microbiology in pilot-scale horizontal subsurface flow constructed wetlands treating domestic wastewater. Ecol Eng 108:70–77. https://doi.org/10.1016/j.ecoleng.2017.08.007
Zheng YL, Jiang XF, Hou LJ, Liu M, Lin XB, Gao J, Li XF, Yin GY, Yu CD, Wang R (2016) Shifts in the community structure and activity of anaerobic ammonium oxidation bacteria along an estuarine salinity gradient. J Geophys Res Biogeosci. https://doi.org/10.1002/2015JG003300
Bai R, Xi D, He JZ, Hu HW, Fang YT, Zhang LM (2015) Activity, abundance and community structure of anammox bacteria along depth profiles in three different paddy soils. Soil Biol Biochem 91:212–221. https://doi.org/10.1016/j.soilbio.2015.08.040
Zhu GB, Wang SY, Wang C, Zhou LG, Zhao SY, Li YX, Li FB, Jetten MSM, Lu YL, Schwark L (2019) Resuscitation of anammox bacteria after > 10,000 years of dormancy. ISME J 13:1098–1109. https://doi.org/10.1038/s41396-018-0316-5
Lipsewers YA, Bale NJ, Hopmans EC, Schouten S, Sinninghe Damste JS, Villanueva L (2014) Seasonality and depth distribution of the abundance and activity of ammonia oxidizing microorganisms in marine coastal sediments (North Sea). Front Microbio 5:472. https://doi.org/10.3389/fmicb.2014.00472
Humbert S, Tarnawski S, Fromin N, Mallet MP, Aragno M, Zopfi J (2010) Molecular detection of anammox bacteria in terrestrial ecosystems: distribution and diversity. ISME J 4:450–454. https://doi.org/10.1038/ismej.2009.125
Srivastava J, Gupta A, Chandra H (2008) Managing water quality with aquatic macrophytes. Rev Environ Sci Biotech 7:255–266. https://doi.org/10.1007/s11157-008-9135-x
Dhote S, Dixit S (2009) Water quality improvement through macrophytes-a review. Environ Monit Assess 152:149–153. https://doi.org/10.1007/s10661-008-0303-9
Cui J, Zhao J, Wang Z, Cao WW, Zhang SH, Liu JM, Bao ZH (2020) Diversity of active root-associated methanotrophs of three emergent plants in a eutrophic wetland in northern China. AMB Expr 10:48. https://doi.org/10.1186/s13568-020-00984-x
Zhao JW, Xu YF, Peng L, Liu GL, Wan XQ, Hua YM, Zhu DW, Hamilton DP (2019) Diversity of anammox bacteria and abundance of functional genes for nitrogen cycling in the rhizosphere of submerged macrophytes in a freshwater lake in summer. J Soil Sediments 19:3648–3656. https://doi.org/10.1007/s11368-019-02340-4
Wang SY, Pi YX, Jiang YY, Pan HW, Wang XX, Wang XM, Zhou JM, Zhu GB (2019) Nitrate reduction in the reed rhizosphere of a riparian zone: from functional genes to activity and contribution. Environ Res. https://doi.org/10.1016/j.envres.2019.108867
Duan XN, Wang XK, Mu YJ, Ouyang ZY (2005) Seasonal and diurnal variations in methane emissions from Wuliangsu Lake in arid regions of China. Atmos Environ 39:4479–4487. https://doi.org/10.1016/j.atmosenv.2005.03.045
Vymazal J (2013) Emergent plants used in free water surface constructed wetlands: a review. Ecol Eng 61:582–592. https://doi.org/10.1016/j.ecoleng.2013.06.023
Zhang SH, Cui J, Zhang M, Liu JM, Wang LX, Zhao J, Bao ZH (2021) Diversity of active anaerobic ammonium oxidation (ANAMMOX) and nirK-type denitrifying bacteria in macrophyte roots in a eutrophic wetland. J Soil Sediment 21:2465–2473. https://doi.org/10.1007/s11368-021-02926-x
Paranychianakis NV, Tsiknia M, Kalogerakis N (2016) Pathways regulating the removal of nitrogen in planted and unplanted subsurface flow constructed wetlands. Water Res 102:321–329. https://doi.org/10.1016/j.watres.2016.06.048
Racchetti E, Longhi D, Ribaudo C, Soana E, Bartoli M (2017) Nitrogen uptake and coupled nitrification-denitrification in riverine sediments with benthic microalgae and rooted macrophytes. Aquat Sci 79:487–505. https://doi.org/10.1007/s00027-016-0512-1
Zhou XH, Zhang JP, Li YM, Liu B, Chu JY, Wang MY, He ZL (2016) Distribution characteristics of ammonia oxidizing microorganisms in rhizosphere sediments of cattail. Ecol eng 88:99–111. https://doi.org/10.1016/j.ecoleng.2015.12.023
Zhou XH, Zhang JP, Wen CZ (2017) Community composition and abundance of anammox bacteria in cattail rhizosphere sediments at three phenological stages. Curr Microbiolo 74:1349–1357. https://doi.org/10.1007/s00284-017-1324-9
Su R, Huang R, Zeng J, Zhan DY, He RJ, Yu ZB, Wu QL (2021) Rhizosphere-associated nosZII microbial community of Phragmites australis and its influence on nitrous oxide emissions in two different regions. J Soils Sediments 21:3326–3341. https://doi.org/10.1007/s11368-021-02967-2
Li H, Yang XR, Weng BS, Su JQ, Nie SA, Gilbert JA, Zhu YG (2016) The phenological stage of rice growth determines anaerobic ammonium oxidation activity in rhizosphere soil. Soil Biol Biochem 100:59–65. https://doi.org/10.1016/j.soilbio.2016.05.015
Zhao DY, Luo J, Zeng J, Wang M, Yan WM, Huang R, Wu QL (2014) Effects of submerged macrophytes on the abundance and community composition of ammonia-oxidizing prokaryotes in a eutrophic lake. Environ Sci Pollut Res 21(1):389–398. https://doi.org/10.1007/s11356-013-1909-1
He YL, Tao WD, Wang ZY, Shayya W (2012) Effects of pH and seasonal temperature variation on simultaneous partial nitrification and anammox in free-water surface wetlands. J Environ Manage 110:103–109. https://doi.org/10.1016/j.jenvman.2012.06.009
Sun HY, Lu XX, Yu RH, Yang J, Liu XY, Cao ZX, Zhang ZZ, Li MX (2021) Geng Y (2021) Eutrophication decreased CO2 but increased CH4 emissions from lake: a case study of a shallow Lake Ulansuhai. Water Res 201:117363. https://doi.org/10.1016/j.watres.2021.117363
Liu JM, Bao ZH, Cao WW, Han JJ, Zhao J, Kang ZZ, Wang LX, Zhao J (2020) Enrichment of type I methanotrophs with nirs genes of three emergent macrophytes in a eutrophic wetland in China. Microbes Environ. https://doi.org/10.1264/jsme2.me19098
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Luo Y, Yuan H, Zhao J, Qi Y, Cao WW, Liu JM, Guo W, Bao ZH (2021) Multiple factors influence bacterial community diversity and composition in soils with rare earth element and heavy metal co-contamination. Ecotox Environ Safe 225:112749. https://doi.org/10.1016/j.ecoenv.2021.112749
Lange M, Eisenhauer N, Sierra CA, Bessler H, Engels C, Griffiths RI, Mellado-Vazquez PG, Malik AA, Roy J, Scheu S, Steinbeiss S, Thomson BC, Trumbore SE, Gleixner G (2015) Plant diversity increases soil microbial activity and soil carbon storage. Nat Commun 6:6707. https://doi.org/10.1038/ncomms7707
Bodelier PLE, Libochant JA, Blom CWPM, Laanbroek HJ (1996) Dynamics of nitrification and denitrification in root-oxygenated sediments and adaptation of ammonia-oxidizing bacteria to low-oxygen or anoxic habitat. Appl Environ Microbiol 62(11):4100–4107. https://doi.org/10.1016/S0027-5107(96)00126-1
Ottosen LDM, Risgaard-Petersen N, Nielsen LP (1999) Direct and indirect measurements of nitrification and denitrification in the rhizosphere of aquatic macrophytes. Aquat Microb Ecol 19:81–91. https://doi.org/10.3354/ame019081
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interations with plants and other organisms. Annu Rev Plant Biol 57:233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Yin XJ, Lu J, Wang YC, Liu GL, Hua YM, Wan XQ, Zhao JW, Zhu DW (2020) The abundance of nirS-type denitrifiers and anammox bacteria in rhizospheres was affected by the organic acids secreted from roots of submerged macrophytes. Chemosphere 240:124903. https://doi.org/10.1016/j.chemosphere.2019.124903
Fu LL, Chen YY, Li SQ, He H, Mi TZ, Zhen Y, Yu ZG (2019) Shifts in the anammox bacterial community structure and abundance in sediments from the changjiang estuary and its adjacent area. Syst Appl Microbiol 42:383–396. https://doi.org/10.1016/j.syapm.2018.12.008
Chu JY, Zhang JP, Zhou XH, Liu B, Li YM (2015) A Comparison of anammox bacterial abundance and community structures in three different emerged plants-related sediments. Curr Microbiol 71(3):421–427. https://doi.org/10.1007/s00284-015-0851-5
Hu JL, Zhou YH, Lei ZY, Liu GL, Hua YM, Zhou WB, Wan XQ, Zhu DW, Zhao JW (2020) Effects of Potamogeton crispus decline in the rhizosphere on the abundance of anammox bacteria and nirS denitrifying bacteria. Environ Pollut 260:114018. https://doi.org/10.1016/j.envpol.2020.114018
Awata T, Oshiki M, Kindaichi T, Ozaki N, Ohashi A, Okabe S (2013) Physiological characterization of an anaerobic ammonium-oxidizing bacterium belonging to the “Candidatus scalindua” group. Appl Environ Microbiol 79:4145–4148. https://doi.org/10.1128/AEM.00056-13
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339. https://doi.org/10.1007/s11104-009-9895-2
Liu D, Fang S, Tian Y, Chang SX (2014) Nitrogen transformations in the rhizosphere of different tree types in a seasonally flooded soil. Plant Soil Environ 60:249–254. https://doi.org/10.1270/jsbbs.64.193
Zheng YL, Hou LJ, Liu M, Yin GY, Gao J, Jiang XF, Lin XB, Li XF, Yu CD, Wang R (2016) Community composition and activity of anaerobic ammonium oxidation bacteria in the rhizosphere of salt-marsh grass Spartina alterniflora. Appl Microbio Biotech 100(18):8203–8212. https://doi.org/10.1007/s00253-016-7625-2
Fu BB, Liu JW, Yang HM, Chang T, Hsu TC, He BY, Dai MH, Kao SJ, Zhao MX, Zhang XH (2015) Shift of anammox bacterial community structure along the Pearl Estuary and the impact of environmental factors. J Geophys Res Oceans 120:2869–2883. https://doi.org/10.1002/2014JC010554
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, MoenneLoccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361. https://doi.org/10.1007/s11104-008-9568-6
Weng BS, Xie XY, Yang JJ, Liu JC, Lu HL, Yan CL (2013) Research on the nitrogen cycle in rhizosphere of Kandelia obovata under ammonium and nitrate addition. Mar Pollut Bull 76:227–240. https://doi.org/10.1016/j.marpolbul.2013.08.034
Chen T, Hu WG, He SB, Zhang X, Niu YH (2020) Diversity and community structure of ammonia-oxidizing archaea in rhizosphere soil of four plant groups in Ebinur Lake wetland. Can J Microbio 67(4):271–280. https://doi.org/10.1139/cjm-2020-0228
Subbarao GV, Ishikawa T, Ito O, Nakahara K, Wang HY, Berry WL (2006) A biolumiuescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiaria humidicola. Plant Soil 288:101–112. https://doi.org/10.1007/s11104-006-9094-3
Yang XR, Li H, Nie SA, Su JQ, Weng BS, Zhu GB, Yao HY, Gilbert JA, Zhu YG (2015) Potential contribution of anammox to nitrogen loss from paddy soils in southern china. Appl Environ Microbiol 81:938–947. https://doi.org/10.1128/AEM.02664-14
Strous M, Kuenen JG, Jetten MS (1999) Key physiology of anaerobic ammonium oxidation. Appl Environ Microbio 65:3248–3250. https://doi.org/10.1128/AEM.65.7.3248-3250.1999
Zhou S, Borjigin S, Riya SH, Terada A, Hosomi M (2014) The relationship between anammox and denitrification in the sediment of an inland river. Sci Total Environ 490:1029–1036. https://doi.org/10.1016/j.scitotenv.2014.05.096
Kumar M, Lin JG (2010) Co-existence of anammox and denitrification for simultaneous nitrogen and carbon removal-Strategies and issues. J Hazard Mater 178:1–9. https://doi.org/10.1016/j.jhazmat.2010.01.077
Funding
This study was funded by the Science and Technology Major Project on Lakes of Inner Mongolia grant (ZDZX2018054), National Natural Science Foundation of China grants (32160028).
Author information
Authors and Affiliations
Contributions
ZSH: Conceptualization, Investigation, Formal analysis, Writing—original draft, Writing—review & editing. ZD: Investigation, Data analysis. GY: Investigation, Data analysis. ZJ: Conceptualization, Investigation, Writing—review&editing. BZH: Conceptualization, Investigation, Formal analysis, Writing—original draft, Writing—review & editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent to Participate
All the authors agree to participate.
Consent for Publication
All the authors agree to submit the paper to Curr Microbiol.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
High-quality anammox hzsB genes high-throughput sequencing data were submitted to GenBank (accession number: SRR12199323-SRR12199326)
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Zhang, D., Guo, Y. et al. Rhizosphere-Associated Anammox Bacterial Diversity and Abundance of Nitrogen Cycle-Related Functional Genes of Emergent Macrophytes in Eutrophic Wetlands. Curr Microbiol 81, 107 (2024). https://doi.org/10.1007/s00284-024-03620-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-024-03620-0