Abstract
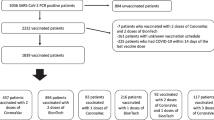
The present work was carried out during the emergence of Delta Variant of Concern (VoC) and aimed to study the change in SARS CoV-2 viral load in Covishield vaccinated asymptomatic/mildly symptomatic health-care workers (HCWs) to find out the optimum isolation period. The SARS CoV-2 viral load was carried out in sequential samples of 55 eligible HCWs which included unvaccinated (UnV; n = 11), single-dose vaccinated (SDV, n = 20) and double-dose vaccinated [DDV, n = 24; short-interval (<6 weeks)] subjects. The mean load of envelope (E) gene on day 5 in SDV [0.42 × 105 copies/reaction] was significantly lower as compared to DDV [6.3 × 105 copies/reaction, P = 0.005] and UnV [6.6 × 105 copies/reaction, P = 0.001] groups. The rate of decline of SARS CoV-2 viral load in the initial 5 days of PCR positivity was significantly higher in SDV as compared to that in DDV (Mean log decline 0.39 vs. 0.19; P < 0.001). This was possibly due to interference of adenoviral immunity of first dose of adenovirus-vectored vaccine in double-dose vaccinated HCWs who had received vaccines within a shorter interval (<6 weeks).


Similar content being viewed by others
Data Availability
Available when required.
Code Availability
NA.
References
Vargese SS, Dev SS, Soman S, Kurian N, Mathew E (2022) Exposure risk and COVID-19 infection among frontline health-care workers: a single tertiary care centre experience. Clin Epidemiol Glob Health 13:100933
Zhou Q, Gao Y, Wang X, Liu R, Du P, Wang X, Zhang X, Lu S, Wang Z, Shi Q (2020) Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med 8(10):629
Evans S, Agnew E, Vynnycky E, Stimson J, Bhattacharya A, Rooney C, Warne B, Robotham J (2021) The impact of testing and infection prevention and control strategies on within-hospital transmission dynamics of COVID-19 in English hospitals. Philos Trans R Soc B 376:20200268
Olmos C, Campaña G, Monreal V, Pidal P, Sanchez N, Airola C, Sanhueza D, Tapia P, Muñoz AM, Corvalan F (2021) SARS-CoV-2 infection in asymptomatic healthcare workers at a clinic in Chile. PLoS ONE 16:e0245913
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383:2603–2615
Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, Ben-Ami R (2021) Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA 325:2457–2465
Acharya CB, Schrom J, Mitchell AM, Coil DA, Marquez C, Rojas S, Wang CY, Liu J, Pilarowski G, Solis L (eds) (2022) Viral load among vaccinated and unvaccinated, asymptomatic and symptomatic persons infected with the SARS-CoV-2 Delta variant. Open forum infectious diseases. Oxford University Press US.
Zuin M, Gentili V, Cervellati C, Rizzo R, Zuliani G (2021) Viral load difference between symptomatic and asymptomatic COVID-19 patients: systematic review and meta-analysis. Infect Dis Rep 13:645–653
15th National Technical Advisory Group on Immunization (NTAGI) (2022) https://main.mohfw.gov.in/sites/default/files/MoM%20NTAGI%20%20May%2028%2C%202021.pdf. Accessed 30 Aug 2022
Strategies to mitigate healthcare personnel staffing shortages (2021) https://www.cdc.gov/coronavirus/2019-ncov/hcp/mitigating-staff-shortages.html. Accessed 30 Aug 2022
Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira IA, Datir R, Collier DA, Albecka A, Singh S (2021) SARS-CoV-2 B. 1.617. 2 Delta variant replication and immune evasion. Nature 599:114–119
Coronavirus Disease 2019 (COVID-19) (2021) Treatment guidelines [Internet]. National Institutes of Health, Bethesda, MD
Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, Schaefer-Babajew DJ, DaSilva J, Muecksch F, Gaebler C (2021) Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med 384:2212–2218
Bhavnani D, James ER, Johnson KE, Beaudenon-Huibregtse S, Chang P, Rathouz PJ, Weldon M, Matouschek A, Young AE (2022) SARS-CoV-2 viral load is associated with risk of transmission to household and community contacts. BMC Infect Dis 22:1–11
Poojary M, Shantaraman A, Jolly B, Scaria V (2020) Computational protocol for assembly and analysis of SARS-nCoV-2 genomes: assembly and analysis of SARS-nCoV-2 genomes. Research Reports 4
Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J (2021) Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596:276–280
Ujjainiya R, Tyagi A, Sardana V, Naushin S, Bhatheja N, Kumar K, Barman J, Prakash S, Kutum R, Bhaskar AK (2022) High failure rate of ChAdOx1-nCoV19 immunization against asymptomatic infection in healthcare workers during a Delta variant surge. Nat Commun 13:1726
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE (2021) Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397:99–111
Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G (2021) Effectiveness of Covid-19 vaccines against the B. 1.617. 2 (Delta) variant. N Engl J Med 385:585–594
16th National Technical Advisory Group on Immunization (NTAGI) Meeting (2021) https://main.mohfw.gov.in/sites/default/files/MoM%20NTAGI%20%20May%2028%2C%202021.pdf. Accessed 30 Aug 2022
Jung J, Kim JY, Park H, Park S, Lim JS, Lim SY, Bae S, Lim Y-J, Kim EO, Kim J (2022) Transmission and infectious SARS-CoV-2 shedding kinetics in vaccinated and unvaccinated individuals. JAMA Netw Open 5:e2213606–e2213606
Mendonça SA, Lorincz R, Boucher P, Curiel DT (2021) Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines 6:97
Ahi YS, Bangari DS, Mittal KS (2011) Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther 11:307–320
Hermosilla E, Coma E, Xie J, Feng S, Cabezas C, Méndez-Boo L, Fina F, Ballo E, Martínez M, Medina-Peralta M (2022) Comparative effectiveness and safety of homologous two-dose ChAdOx1 versus heterologous vaccination with ChAdOx1 and BNT162b2. Nat Commun 13:1639
Purushotham JN, van Doremalen N, Munster VJ (2021) SARS-CoV-2 vaccines: anamnestic response in previously infected recipients. Cell Res 31:827–828
Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, Flaxman A, Wright D, Bellamy D, Bittaye M (2021) T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med 27:270–278
Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G (2020) Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 396:1979–1993
Revised guidelines for home isolation of mild/asymptomatic COVID-19 cases (2022) https://www.mohfw.gov.in/pdf/RevisedIllustratedGuidelinesforHomeIsolationofMildAsymptomaticCOVID19Cases.pdf. Accessed 30 Aug 2023
Funding
None to declare.
Author information
Authors and Affiliations
Contributions
Conceptualization, data analysis and writing manuscript—AG; Methodology (Real-time PCRs)—PK, Sample collection and record maintenance—MR, Recruitment of vaccinated and unvaccinated subjects—RM, Result reporting and manuscript review and editing—KG, Communication with study subjects and their follow-up—RK, Clinical investigation of the study subjects and final decision on their health status—VS, Epidemiological data collection and communication with HCWs for recruitment in the study—PVML, Epidemiological data collection—Manisha & Chanderkanta, Analysis of NGS data—PR, Sample preparation and NGS testing of samples—KP, Quality control of NGS samples and checking the appropriateness of the samples sent for NGS—TD, Overall project administration and editing of manuscript—MPS.
Corresponding author
Ethics declarations
Conflict of interest
Nothing to declare.
Ethical Approval and Consent to Participate
The study was approved by institutional ethics committee (IEC no. Int/IEC/2021/SPL-949 dated 10.06.2021.
Consent for Publication
NA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosh, A., Kanta, P., Ramola, M. et al. Rapid Decline of SARS-CoV-2 Viral Load in Single vs. Double-Dose (Short-Interval <6 Weeks) ChAdOx nCoV-19 Vaccinated Health-Care Workers. Curr Microbiol 81, 95 (2024). https://doi.org/10.1007/s00284-023-03603-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03603-7