Abstract
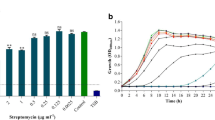
Pseudomonas aeruginosa is a medically important opportunistic pathogen due to its intrinsic ability to form biofilms on different surfaces as one of the defense mechanisms for survival. The fact that it can form biofilms on various medical implants makes it more harmful clinically. Although various antibiotics are used to treat Pseudomonas aeruginosa infections, studies have shown that sub-MIC levels of antibiotics could induce Pseudomonas biofilm formation. The present study thus explored the effect of the aminoglycoside antibiotic gentamicin on the biofilm dynamics of two Pseudomonas aeruginosa strains KPW.1-S1 and HRW.1-S3. Biofilm formation was found to be increased in the presence of increased concentrations of gentamicin. Confocal, scanning electron microscopy, and other biochemical tests deduced that biofilm-forming components exoproteins, eDNA, and exolipids as exopolymeric substances in Pseudomonas aeruginosa biofilms were increased in the presence of gentamicin. An increase in reactive oxygen species generation along with increased cell surface hydrophobicity was also seen for both strains when treated with gentamicin. The observed increase in the adherence of the cells accompanied by the increase in the components of exopolymeric substances may have largely contributed to the increased biofilm production by the Pseudomonas aeruginosa strains under the stress of the antibiotic treatment.





Similar content being viewed by others
Availability of Data and Materials
In this study, all data generated or analyzed are included in the article.
References
Joffe AM, Volpel K, Kibsey PC, Paranchych W (1992) Epidemiological studies of nosocomial infections with Pseudomonas aeruginosa using a DNA probe. Can J Infectious Diseas 3(6):299–299. https://doi.org/10.1155/1992/809648
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2(2):95–108. https://doi.org/10.1038/nrmicro821
Hoyle BD, Costerton JW (1991) Bacterial resistance to antibiotics: the role of biofilms. Prog Drug Res 37:91–105. https://doi.org/10.1007/978-3-0348-7139-6_2
Potera C (1999) Forging a link between biofilms and disease. Science (New York, NY) 283(5409):1837–1839. https://doi.org/10.1126/SCIENCE.283.5409.1837
Domenech M, Ramos-Sevillano E, García E, Moscoso M, Yuste J (2013) Biofilm formation avoids complement immunity and phagocytosis of Streptococcus pneumoniae. Infect Immun 81(7):2606–2615. https://doi.org/10.1128/IAI.00491-13
Schembri MA, Givskov M, Klemm P (2002) An attractive surface: gram-negative bacterial biofilms. Sci STKE. https://doi.org/10.1126/STKE.2002.132.RE6
Santos APA, Watanabe E, de Andrade D (2011) Biofilm on artificial pacemaker: fiction or reality? Arq Bras Cardiol 97(5):e113–e120. https://doi.org/10.1590/S0066-782X2011001400018
Malic S, Hill KE, Playle R, Thomas DW, Williams DW (2011) In vitro interaction of chronic wound bacteria in biofilms. J Wound Care 20(12):569–570. https://doi.org/10.12968/jowc.2011.20.12.569
Costa OYA, Raaijmakers JM, Kuramae EE (2018) Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol. https://doi.org/10.3389/FMICB.2018.01636
Fang FC, Frawley ER, Tapscott T, Vázquez-Torres A (2016) Bacterial stress responses during host infection. Cell Host Microbe 20(2):133–133. https://doi.org/10.1016/J.CHOM.2016.07.009
Yang L, Barken KB, Skindersoe ME, Christensen AB, Givskov M, Tolker-Nielsen T (2007) Effects of iron on DNA release and biofilm development by Pseudomonas aeruginosa. Microbiology 153(5):1318–1328. https://doi.org/10.1099/MIC.0.2006/004911-0
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284(5418):1318–1322. https://doi.org/10.1126/science.284.5418.1318
Shlaes DM, Bradford PA (2018) Antibiotics—from there to where? How the antibiotic miracle is threatened by resistance and a broken market and what we can do about it. Pathogens Immun 3(1):19–19. https://doi.org/10.20411/PAI.V3I1.231
Penesyan A, Paulsen IT, Gillings MR, Kjelleberg S, Manefield MJ (2020) Secondary effects of antibiotics on microbial biofilms. Front Microbiol. https://doi.org/10.3389/fmicb.2020.02109
Lewis K (2005) Persister cells and the riddle of biofilm survival. Biochem Biokhimiia 70(2):267–274. https://doi.org/10.1007/S10541-005-0111-6
Lima MR, Ferreira GF, Nunes Neto WR, Monteiro JdM, Santos ÁRC, Tavares PB, Denadai ÂML, Bomfim MRQ, dos Santos VL, Marques SG, de Souza MA (2019) Evaluation of the interaction between polymyxin B and Pseudomonas aeruginosa biofilm and planktonic cells: reactive oxygen species induction and zeta potential. BMC Microbiol 19(1):115. https://doi.org/10.1186/s12866-019-1485-8
Da Cruz Nizer WS, Inkovskiy V, Versey Z, Strempel N, Cassol E, Overhage J (2021) Oxidative stress response in Pseudomonas aeruginosa. Pathogens 10(9):1187. https://doi.org/10.3390/pathogens10091187
Čáp M, Váchová L, Palková Z (2012) Reactive oxygen species in the signaling and adaptation of multicellular microbial communities. Oxid Med Cell Longev 2012:1–13. https://doi.org/10.1155/2012/976753
McDonnell G, Russell AD (1999) Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12(1):147–147. https://doi.org/10.1128/CMR.12.1.147
Fitzgerald KA, Davies A, Russell AD (1992) Effect of chlorhexidine and phenoxyethanol on cell surface hydrophobicity of Gram-positive and Gram-negative bacteria. Lett Appl Microbiol 14(3):91–95. https://doi.org/10.1111/J.1472-765X.1992.TB00656.X
Dasgupta D, Ghosh R, Sengupta TK (2013) Biofilm-mediated enhanced crude oil degradation by newly isolated pseudomonas species. ISRN Biotechnol 2013:1–13. https://doi.org/10.5402/2013/250749
Bushnell LD, Haas HF (1941) The utilization of certain hydrocarbons by microorganisms. J Bacteriol 41(5):653–673. https://doi.org/10.1128/jb.41.5.653-673.1941
Essar DW, Eberly L, Hadero A, Crawford IP (1990) Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol 172(2):884–900. https://doi.org/10.1128/jb.172.2.884-900.1990
Zegans ME, Wagner JC, Cady KC, Murphy DM, Hammond JH, O’Toole GA (2009) Interaction between Bacteriophage DMS3 and Host CRISPR region inhibits group behaviors of Pseudomonas aeruginosa. J Bacteriol 191(1):210–210. https://doi.org/10.1128/JB.00797-08
Merritt JH, Kadouri DE, O’Toole GA (2006) Growing and analyzing static biofilms. Curr Protoc Microbiol. https://doi.org/10.1002/9780471729259.mc01b01s00
Allan VJ, Callow ME, Macaskie LE, Paterson-Beedle M (2002) Effect of nutrient limitation on biofilm formation and phosphatase activity of a Citrobacter sp. Microbiology (Reading) 148(Pt 1):277–288. https://doi.org/10.1099/00221287-148-1-277
Xie F, Zhang Y, Li G, Zhou L, Liu S, Wang C (2013) The ClpP protease is required for the stress tolerance and biofilm formation in Actinobacillus pleuropneumoniae. PLoS ONE 8(1):e53600. https://doi.org/10.1371/journal.pone.0053600
Nakao R, Ramstedt M, Wai SN, Uhlin BE (2012) Enhanced biofilm formation by Escherichia coli LPS mutants defective in hep biosynthesis. PLoS ONE 7(12):e51241–e51241. https://doi.org/10.1371/JOURNAL.PONE.0051241
Bonasera V, Alberti S, Sacchetti A (2007) Protocol for high-sensitivity/long linear-range spectrofluorimetric DNA quantification using ethidium bromide. Biotechniques 43(2):173–176. https://doi.org/10.2144/000112500
Chen W, Zhang C, Song L, Sommerfeld M, Hu Q (2009) A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Methods 77(1):41–47. https://doi.org/10.1016/J.MIMET.2009.01.001
Wu J, Xi C (2009) Evaluation of different methods for extracting extracellular DNA from the biofilm matrix. Appl Environ Microbiol 75(16):5390–5395. https://doi.org/10.1128/AEM.00400-09
Ciofu O, Rojo-Molinero E, Macià MD, Oliver A (2017) Antibiotic treatment of biofilm infections. APMIS 125(4):304–319. https://doi.org/10.1111/apm.12673
Vincent JL (2003) Nosocomial infections in adult intensive-care units. Lancet (London, England) 361(9374):2068–2077. https://doi.org/10.1016/S0140-6736(03)13644-6
Andersson DI, Hughes D (2014) Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12(7):465–478. https://doi.org/10.1038/nrmicro3270
Song T, Duperthuy M, Wai S (2016) Sub-optimal treatment of bacterial biofilms. Antibiotics 5(2):23. https://doi.org/10.3390/antibiotics5020023
Kaplan JB (2011) Antibiotic-induced biofilm formation. Int J Artif Organs 34(9):737–751. https://doi.org/10.5301/IJAO.5000027
Kumar M, Rao M, Mathur T, Barman TK, Joshi V, Chaira T, Singhal S, Pandya M, Al Khodor S, Upadhyay DJ, Masuda N (2021) Azithromycin exhibits activity against Pseudomonas aeruginosa in chronic rat lung infection model. Front Microbiol. https://doi.org/10.3389/fmicb.2021.603151
Chiang WC, Nilsson M, Jensen PØ, Høiby N, Nielsen TE, Givskov M, Tolker-Nielsen T (2013) Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 57(5):2352–2361. https://doi.org/10.1128/AAC.00001-13
Lewenza S (2013) Extracellular DNA-induced antimicrobial peptide resistance mechanisms in Pseudomonas aeruginosa. Front Microbiol. https://doi.org/10.3389/fmicb.2013.00021
Mulcahy H, Charron-Mazenod L, Lewenza S (2008) Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog 4(11):1000213–1000213. https://doi.org/10.1371/journal.ppat.1000213
Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS (2002) Extracellular DNA required for bacterial biofilm formation. Science 295(5559):1487–1487. https://doi.org/10.1126/science.295.5559.1487
Tahrioui A, Duchesne R, Bouffartigues E, Rodrigues S, Maillot O, Tortuel D, Hardouin J, Taupin L, Groleau MC, Dufour A, Déziel E, Brenner-Weiss G, Feuilloley M, Orange N, Lesouhaitier O, Cornelis P, Chevalier S (2019) Extracellular DNA release, quorum sensing, and PrrF1/F2 small RNAs are key players in Pseudomonas aeruginosa tobramycin-enhanced biofilm formation. NPJ Biofilms and Microbiomes. https://doi.org/10.1038/s41522-019-0088-3
Zhang C, Wang C, Xiu Z (2021) Regulation of c-di-GMP in biofilm formation of klebsiella pneumoniae in response to antibiotics and probiotic supernatant in a chemostat system. Curr Microbiol 78(1):133–143. https://doi.org/10.1007/s00284-020-02258-y
Hu H, Ramezanpour M, Hayes A, Liu S, Psaltis AJ, Wormald P, Vreugde S (2019) Sub-inhibitory clindamycin and azithromycin reduce s. aureus exoprotein induced toxicity, inflammation, barrier disruption and invasion. J Clin Med 8(10):1617. https://doi.org/10.3390/jcm8101617
Zarnowski R, Westler WM, Lacmbouh GA, Marita JM, Bothe JR, Bernhardt J, Sahraoui ALH, Fontainei J, Sanchez H, Hatfeld RD, Ntambi JM, Nett JE, Mitchell AP, Andes DR (2014) Novel entries in a fungal biofilm matrix encyclopedia. MBio 5(4):1–13. https://doi.org/10.1128/mBio.01333-14
Nwodo UU, Green E, Okoh AI (2012) Bacterial exopolysaccharides: functionality and prospects. Int J Mol Sci 13:14002–14015. https://doi.org/10.3390/ijms131114002
Laverty G, Gorman SP, Gilmore BF (2014) Biomolecular mechanisms of Pseudomonas aeruginosa and Escherichia coli biofilm formation. Pathogens (Basel, Switzerland) 3(3):596–632. https://doi.org/10.3390/PATHOGENS3030596
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Publ Group. https://doi.org/10.1038/nrmicro2415
Smirnova GV, Samoylova ZY, Muzyka NG, Oktyabrsky ON (2009) Influence of polyphenols on Escherichia coli resistance to oxidative stress. Free Radical Biol Med 46(6):759–768. https://doi.org/10.1016/J.FREERADBIOMED.2008.11.017
Acknowledgements
All authors would like to acknowledge Ritabrata Ghosh and Kashinath Sahu for their technical support of confocal laser scanning and scanning electron microscopy, respectively.
Funding
The study was funded by IISER Kolkata.
Author information
Authors and Affiliations
Contributions
AK and TKS conceived the study and designed the experiments. AK performed most of the experiments. SKS performed experiments on ROS generation and cell surface hydrophobicity. AK, SKS, PB, KP, and TKS analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical Approval
Not applicable.
Consent to Participate
All authors had consented to participate in the study.
Consent for Publication
All authors have given consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1
(DOCX 3145 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, A., Saha, S.K., Banerjee, P. et al. Antibiotic-Induced Biofilm Formations in Pseudomonas aeruginosa Strains KPW.1-S1 and HRW.1-S3 are Associated with Increased Production of eDNA and Exoproteins, Increased ROS Generation, and Increased Cell Surface Hydrophobicity. Curr Microbiol 81, 11 (2024). https://doi.org/10.1007/s00284-023-03495-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03495-7