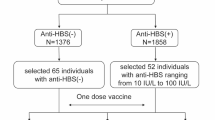
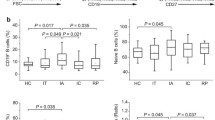
Abstract
Vaccination is the most effective way to prevent Hepatitis B (HB) infection. The goal of vaccination is to induce immunological memory. Hence, determining the frequency of memory B-cell (MBC) subsets is an important indicator of vaccine efficacy. This study aimed to evaluate the frequency of different B-cell subpopulations and the expression of PD-1 on B-cell subsets in low responders (LR) and high responders (HR) to HB vaccine. According to our findings, the expression level of PD-1 was significantly higher on atypical MBC (atMBC) than that of naive B cell and classical MBC (cMBC) in LR and HR groups. Moreover, cMBCs had a significant higher PD-1 expression than naive B cells in LR group. No significant differences were found in the frequency of various B-cell subpopulations and the expression level of PD-1 on B-cell subsets between LR and HR groups. We observed a negative correlation between age and HBsAb titer and a positive correlation between age and PD-1 expression level on cMBC in LR group. It can be concluded that inadequate specific memory B-cell response, rather than total memory B-cell deficiency, may be implicated in low responsive rate to HB vaccine in healthy individuals.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Lai A, Sagnelli C, Lo Presti A, Cella E, Angeletti S, Spoto S et al (2018) What is changed in HBV molecular epidemiology in Italy? J Med Virol 90(5):786–795. https://doi.org/10.1002/jmv.25027
Chemin I, Pujol FH (2022) Special issue: “updates on HBV infection”. Microorganisms 10(3): 580. https://doi.org/10.3390/microorganisms10030580
Kumar M, Pahuja S, Khare P, Kumar A (2023) Current challenges and future perspectives of diagnosis of hepatitis B virus. Diagnostics (Basel) 13(3): 368. https://doi.org/10.3390/diagnostics13030368.
Stasi C, Silvestri C, Voller F (2017) Emerging trends in epidemiology of hepatitis B virus infection. J Clin Transl Hepatol 5(3):272. https://doi.org/10.14218/JCTH.2017.00010
Doedée A, Kannegieter N, Öztürk K, van Loveren H, Janssen R, Buisman A-M (2016) Higher numbers of memory B-cells and Th2-cytokine skewing in high responders to hepatitis B vaccination. Vaccine 34(19):2281–2289. https://doi.org/10.1016/j.vaccine.2015.12.027
Borzooy Z, Streinu-Cercel A, Mirshafiey A, Khamseh A, Mahmoudie MK, Navabi SS et al (2016) IL-17 and IL-22 genetic polymorphisms in HBV vaccine non-and low-responders among healthcare workers. Germs 6(1):14. https://doi.org/10.11599/germs.2016.1084
Kao J-H (2015) Hepatitis B vaccination and prevention of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 29(6):907–917. https://doi.org/10.1016/j.bpg.2015.09.011
Hummel IB, Zitzmann A, Erl M, Wenzel JJ, Jilg W (2016) Characteristics of immune memory 10–15 years after primary hepatitis B vaccination. Vaccine 34(5):636–642. https://doi.org/10.1016/j.vaccine.2015.12.033
Seifert M, Küppers R (2016) Human memory B cells. Leukemia 30(12):2283–2292. https://doi.org/10.1038/leu.2016.226
Sebina I, Pepper M (2018) Humoral immune responses to infection: common mechanisms and unique strategies to combat pathogen immune evasion tactics. Curr Opin Immunol 51:46–54. https://doi.org/10.1016/j.coi.2018.02.001
Peeridogaheh H, Meshkat Z, Habibzadeh S, Arzanlou M, Shahi JM, Rostami S et al (2018) Current concepts on immunopathogenesis of hepatitis B virus infection. Virus Res 245:29–43. https://doi.org/10.1016/j.virusres.2017.12.007
Bertoletti A, Ferrari C (2016) Adaptive immunity in HBV infection. J Hepatol 64(1):S71–S83. https://doi.org/10.1016/j.jhep.2016.01.026
Kusumoto S, Tanaka Y, Ueda R, Mizokami M (2011) Reactivation of hepatitis B virus following rituximab-plus-steroid combination chemotherapy. J Gastroenterol 46(1):9–16. https://doi.org/10.1007/s00535-010-0331-4
Imkeller K, Wardemann H (2018) Assessing human B cell repertoire diversity and convergence. Immunol Rev 284(1):51–66. https://doi.org/10.1111/imr.12670
Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P et al (2013) Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol 190(3):1038–1047. https://doi.org/10.4049/jimmunol.1202438
Hu Z, Luo Z, Wan Z, Wu H, Li W, Zhang T et al (2015) HIV-associated memory B cell perturbations. Vaccine 33(22):2524–2529. https://doi.org/10.1016/j.vaccine.2015.04.008
Salimzadeh L, Le Bert N, Dutertre C-A, Gill US, Newell EW, Frey C et al (2018) PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection. J Clin Investig 128(10):4573–4587. https://doi.org/10.1172/JCI121957
Rath S, Devey M (1988) IgG subclass composition of antibodies to HBsAg in circulating immune complexes from patients with hepatitis B virus infections. Clin Exp Immunol 72(1):164–167
Pupovac A, Good-Jacobson KL (2017) An antigen to remember: regulation of B cell memory in health and disease. Curr Opin Immunol 45:89–96. https://doi.org/10.1016/j.coi.2017.03.004
Portugal S, Obeng-Adjei N, Moir S, Crompton PD, Pierce SK (2017) Atypical memory B cells in human chronic infectious diseases: an interim report. Cell Immunol 321:18–25. https://doi.org/10.1016/j.cellimm.2017.07.003
Akkaya M, Kwak K, Pierce SK (2020) B cell memory: building two walls of protection against pathogens. Nat Rev Immunol 20(4):229–238. https://doi.org/10.1038/s41577-019-0244-2
Burton AR, Maini MK (2021) Human antiviral B cell responses: emerging lessons from hepatitis B and COVID-19. Immunol Rev 299(1):108–117. https://doi.org/10.1111/imr.12953
Milich DR, Leroux-Roels GG (2003) Immunogenetics of the response to HBsAg vaccination. Autoimmun Rev 2(5):248–257. https://doi.org/10.1016/s1568-9972(03)00031-4
Rosado MM, Scarsella M, Pandolfi E, Cascioli S, Giorda E, Chionne P et al (2011) Switched memory B cells maintain specific memory independently of serum antibodies: the hepatitis B example. Eur J Immunol 41(6):1800–1808. https://doi.org/10.1002/eji.201041187
Shokrgozar MA, Mahmoodzadeh-Niknam H, Shokri F (2002) Distribution of circulating immune cells in responder and non-responder individuals to hepatitis B vaccine. Iran Biomed J 6(1):1–5
Shokrgozar MA, Shokri F (2001) Enumeration of hepatitis B surface antigen-specific B lymphocytes in responder and non-responder normal individuals vaccinated with recombinant hepatitis B surface antigen. Immunology 104(1):75–79. https://doi.org/10.1046/j.1365-2567.2001.01273.x
Lømo J, Blomhoff HK, Jacobsen SE, Krajewski S, Reed JC, Smeland EB (1997) Interleukin-13 in combination with CD40 ligand potently inhibits apoptosis in human B lymphocytes: upregulation of Bcl-xL and Mcl-1. Blood J Am Soc Hematol 89(12):4415–4424
Doi H, Yoshio S, Yoneyama K, Kawai H, Sakamoto Y, Shimagaki T, et al (2019) Immune determinants in the acquisition and maintenance of antibody to hepatitis B surface antigen in adults after first‐time hepatitis B vaccination. Hepatol Commun 3(6): 812–824. https://doi.org/10.1002/hep4.1357
Tsai Y-F, Yang C-I, Du J-S, Lin M-H, Tang S-H, Wang H-C et al (2019) Rituximab increases the risk of hepatitis B virus reactivation in non-Hodgkin lymphoma patients who are hepatitis B surface antigen-positive or have resolved hepatitis B virus infection in a real-world setting: a retrospective study. PeerJ 7: e7481. https://doi.org/10.7717/peerj.7481
Garner-Spitzer E, Wagner A, Paulke-Korinek M, Kollaritsch H, Heinz FX, Redlberger-Fritz M et al (2013) Tick-borne encephalitis (TBE) and hepatitis B nonresponders feature different immunologic mechanisms in response to TBE and influenza vaccination with involvement of regulatory T and B cells and IL-10. J Immunol 191(5):2426–2436. https://doi.org/10.4049/jimmunol.1300293
Burton AR, Pallett LJ, McCoy LE, Suveizdyte K, Amin OE, Swadling L et al (2018) Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J Clin Investig 128(10):4588–4603. https://doi.org/10.1172/JCI121960
Knox JJ, Buggert M, Kardava L, Seaton KE, Eller MA, Canaday DH, et al (2017) T-bet+ B cells are induced by human viral infections and dominate the HIV gp140 response. JCI Insight 2(8). https://doi.org/10.1172/jci.insight.92943
Jubel JM, Barbati ZR, Burger C, Wirtz DC, Schildberg FA (2020) The role of PD-1 in acute and chronic infection. Front Immunol 11:487. https://doi.org/10.3389/fimmu.2020.00487
Amu S, Lavy-Shahaf G, Cagigi A, Hejdeman B, Nozza S, Lopalco L et al (2014) Frequency and phenotype of B cell subpopulations in young and aged HIV-1 infected patients receiving ART. Retrovirology 11(1):1–13. https://doi.org/10.1186/s12977-014-0076-x
Wilson JK, Zhao Y, Singer M, Spencer J, Shankar-Hari M (2018) Lymphocyte subset expression and serum concentrations of PD-1/PD-L1 in sepsis-pilot study. Crit Care 22(1):1–7. https://doi.org/10.1186/s13054-018-2020-2
Lu Y, Zhu Q, Li Y, Wang Q, Jiang C, Li Z et al (2020) Aberrant expression of PD-1 on B cells and its association with the clinical parameters of systemic lupus erythematosus. https://doi.org/10.21203/rs.2.17942/v2
Poonia B, Ayithan N, Nandi M, Masur H, Kottilil S (2018) HBV induces inhibitory FcRL receptor on B cells and dysregulates B cell-T follicular helper cell axis. Sci Rep 8(1):1–14. https://doi.org/10.1038/s41598-018-33719-x
Thibult M-L, Mamessier E, Gertner-Dardenne J, Pastor S, Just-Landi S, Xerri L et al (2013) PD-1 is a novel regulator of human B-cell activation. Int Immunol 25(2):129–137. https://doi.org/10.1093/intimm/dxs098
Roe K, Shu GL, Draves KE, Giordano D, Pepper M, Clark EA (2019) Targeting antigens to CD180 but not CD40 programs immature and mature B cell subsets to become efficient APCs. J Immunol 203(7):1715–1729. https://doi.org/10.4049/jimmunol.1900549
Chung JB, Silverman M, Monroe JG (2003) Transitional B cells: step by step towards immune competence. Trends Immunol 24(6):342–348. https://doi.org/10.1016/s1471-4906(03)00119-4
Perez-Andres M, Paiva B, Nieto WG, Caraux A, Schmitz A, Almeida J et al (2010) Human peripheral blood B-cell compartments: a crossroad in B-cell traffic. Cytometry B Clin Cytom 78(S1):S47–S60. https://doi.org/10.1002/cyto.b.20547
Weinberg A, Lindsey J, Bosch R, Persaud D, Sato P, Ogwu A et al (2018) B and T cell phenotypic profiles of African HIV-infected and HIV-exposed uninfected infants: associations with antibody responses to the pentavalent rotavirus vaccine. Front Immunol 8:2002. https://doi.org/10.3389/fimmu.2017.02002
Schmidlin H, Diehl SA, Blom B (2009) New insights into the regulation of human B-cell differentiation. Trends Immunol 30(6):277–285. https://doi.org/10.1016/j.it.2009.03.008
Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB (2019) Immunosenescence and human vaccine immune responses. Immun Ageing 16(1):1–16. https://doi.org/10.1186/s12979-019-0164-9
Notarte KI, Ver AT, Velasco JV, Pastrana A, Catahay JA, Salvagno GL et al (2021) Effects of age, sex, serostatus and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech vaccination: a systematic review. medRxiv. https://doi.org/10.1080/10408363.2022.2038539
Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R et al (2021) Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. MedRxiv. https://doi.org/10.1093/cid/ciab381
Colonna-Romano G, Bulati M, Aquino A, Pellicano M, Vitello S, Lio D et al (2009) A double-negative (IgD− CD27−) B cell population is increased in the peripheral blood of elderly people. Mech Ageing Dev 130(10):681–690. https://doi.org/10.1016/j.mad.2009.08.003
Dunn-Walters DK, Stewart AT, Sinclair EL, Serangeli I (2020) Age-related changes in B cells relevant to vaccine responses. Interdiscip Top Gerontol Geriatr 43:56–72. https://doi.org/10.1159/000504479
Ubillos I, Campo JJ, Requena P, Ome-Kaius M, Hanieh S, Rose H et al (2017) Chronic exposure to malaria is associated with inhibitory and activation markers on atypical memory B cells and marginal zone-like B cells. Front Immunol 8:966. https://doi.org/10.3389/fimmu.2017.00966
Yang S, Tian G, Cui Y, Ding C, Deng M, Yu C et al (2016) Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep 6(1):1–12. https://doi.org/10.1038/srep27251
Acknowledgements
The authors wish thank to all the individuals who consented to participate in this study.
Funding
This study was a part of MSc project of Zahra Saleh, Department of Immunology Shiraz University of Medical Sciences and was financially supported by Shiraz University of Medical Sciences (Grant no 21740).
Author information
Authors and Affiliations
Contributions
KK designed the project, ZS performed the experiments, analyzed the data, prepared all figures and tables, and wrote the manuscript; KK, FM, MA, MH, and DK reviewed and edited the manuscript. All authors have read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study protocol was approved by the Ethics Committee of the Shiraz University of Medical Sciences (IR.SUMS.REC.1399.907).
Informed Consent
Written informed consent was obtained from all the participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saleh, Z., Mehdipour, F., Ataollahi, M.R. et al. Frequency of B-Cell Subpopulations in Low Responders in Comparison with High Responders to Hepatitis B Vaccine Among Health Care Workers. Curr Microbiol 80, 296 (2023). https://doi.org/10.1007/s00284-023-03367-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03367-0