Abstract
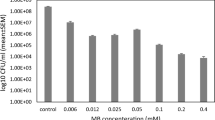
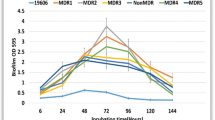
Drug-resistant biofilm producer A. baumannii isolates are a global concern that warns researchers about the development of new treatments. This study was designed to analyze the effect of photodynamic therapy (PDT) as monotherapy and associated with melittin on multidrug-resistant A. baumannii isolates. Sub-lethal doses of photosensitizer, LED, and PDT were determined. The PDT effect on the biofilm and expression of biofilm-associated genes was evaluated by scanning electron microscopy and quantitative real-time PCR (qRT-PCR) methods, respectively. The synergistic effect of PDT and melittin on the survival of MDR/XDR strong biofilm producer isolates was evaluated by checkerboard assay. Survival rates were significantly decreased at the lowest concentration of 12.5–50 μg/ml in 4 min at an energy density of 93.75 J/cm2 (P < 0.05). The optimized PDT method had a bactericidal effect against all tested groups, and the mean expression levels of csu, abaI, bap, and ompA genes in the strong biofilm producers were decreased significantly compared to the control group. The combined effect of LED and melittin successfully reduced the MDR/XDR A. baumannii strong biofilm producers' growth from 3.1 logs. MB-mediated aPDT and combined treatment of PDT with melittin, which has been investigated for the first time in this study, can be an efficient strategy against MDR/XDR A. baumannii isolates with strong biofilm production capacity.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Ayoub Moubareck C, Hammoudi Halat D (2020) Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics 9(3):119. https://doi.org/10.3390/antibiotics9030119
Eze EC, Chenia HY, El Zowalaty ME (2018) Acinetobacter baumannii biofilms: effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect Drug Resist 11:2277–2299. https://doi.org/10.2147/IDR.S169894
Colquhoun JM, Rather PN (2020) Insights into mechanisms of biofilm formation in Acinetobacter baumannii and implications for uropathogenesis. Front Cell Infect Microbiol 10:253. https://doi.org/10.3389/fcimb.2020.00253
Breslawec AP, Wang S, Li C et al (2021) Anionic amino acids support hydrolysis of poly-β-(1, 6)-N-acetylglucosamine exopolysaccharides by the biofilm dispersing glycosidase Dispersin. B J Biol Chem 296:100203. https://doi.org/10.1074/jbc.RA120.015524
Loehfelm TW, Luke NR, Campagnari AA (2008) Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol 190(3):1036–1044. https://doi.org/10.1128/JB.01416-07
Pourhajibagher M et al (2020) Photodisinfection effects of silver sulfadiazine nanoliposomes doped-curcumin on Acinetobacter baumannii: a mouse model. Nanomedicine 15(05):437–452. https://doi.org/10.2217/nnm-2019-0315
Chen J, Fan T, Xie Z et al (2020) Advances in nanomaterials for photodynamic therapy applications: status and challenges. Biomaterials 237:119827. https://doi.org/10.1016/j.biomaterials.2020.119827
Kashef N, Abadi GRS, Djavid GE (2012) Phototoxicity of phenothiazinium dyes against methicillin-resistant Staphylococcus aureus and multi-drug resistant Escherichia coli. Photodiagnosis Photodyn Ther 9(1):11–15. https://doi.org/10.1016/j.pdpdt.2011.11.004
Boluki E, Moradi M, Azar PS et al (2019) The effect of antimicrobial photodynamic therapy against virulence genes expression in colistin-resistance Acinetobacter baumannii. Laser Ther 28(1):27–33. https://doi.org/10.5978/islsm.28_19-OR-03
Perez F, Hujer AM, Hujer KM et al (2007) Global challenge of multidrug-resistant Acinetobacter baumannii. Agents Chemother 51(10):3471–3484. https://doi.org/10.1128/AAC.01464-06
Wozniak A, Rapacka-Zdonczyk A, Mutters NT et al (2019) Antimicrobials are a photodynamic inactivation adjuvant for the eradication of extensively drug-resistant Acinetobacter baumannii. Front Microbiol 10:229. https://doi.org/10.3389/fmicb.2019.00229
Akbari R, Hakemi-Vala M, Pashaie F et al (2019) Highly synergistic effects of melittin with conventional antibiotics against multidrug-resistant isolates of Acinetobacter baumannii and Pseudomonas aeruginosa. Microb Drug Resist 25(2):193–202. https://doi.org/10.1089/mdr.2018.0016
Galdiero E, Siciliano A, Gesuele R et al (2019) Melittin inhibition and eradication activity for resistant polymicrobial biofilm isolated from a dairy industry after disinfection. Int J Microbiol 15:4012394. https://doi.org/10.1155/2019/4012394
Alni RH, Tavasoli F, Barati A et al (2020) Synergistic activity of melittin with mupirocin: a study against methicillin-resistant S. Aureus (MRSA) and methicillin-susceptible S. Aureus (MSSA) isolates. Saudi J Biol Sci 27(10):2580–2585. https://doi.org/10.1016/j.sjbs.2020.05.027
Brown A, Smith H (2015) Benson’s microbiological applications: laboratory manual in general microbiology, short version, 13th edn. McGraw-Hill Education, New York, pp 257–261
Woodford N, Ellington MJ, Coelho JM et al (2006) Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 27(4):351–353. https://doi.org/10.1016/j.ijantimicag.2006.01.004
CLSI (2016) Performance standards for antimicrobial susceptibility testing. Clin Lab Stand Inst 35(3):16–38
Magiorakos AP, Srinivasan A, Carey RB et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3):268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x
Tendolkar PM, Baghdayan AS, Gilmore MS et al (2004) Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect Immun 72(10):6032–6039. https://doi.org/10.1128/IAI.72.10.6032-6039.2004
Ghasemi E, Zhang S, Mi P et al (2018) Phenotypic and genotypic investigation of biofilm formation in clinical and environmental isolates of Acinetobacter baumannii. Arch Clin Infect Dis 13(4):e12914. https://doi.org/10.5812/archcid.12914
Seifi K, Kazemian H, Heidari H et al (2016) Evaluation of biofilm formation among Klebsiella pneumoniae isolates and molecular characterization by ERIC-PCR. Jundishapur J Microbiol 9(1):e30682. https://doi.org/10.5812/jjm.30682
Mahmoudi H, Pourhajibagher M, Alikhani MY et al (2019) The effect of antimicrobial photodynamic therapy on the expression of biofilm associated genes in Staphylococcus aureus strains isolated from wound infections in burn patients. Photodiagnosis Photodyn Ther 25:406–413. https://doi.org/10.1016/j.pdpdt.2019.01.028
Man A, Santacroce L, Jacob R et al (2019) Antimicrobial activity of six essential oils against a group of human pathogens: a comparative study. Pathogens 8(1):15. https://doi.org/10.3390/pathogens8010015
Alni RH, Ghorban K, Dadmanesh M (2020) Combined effects of Allium sativum and Cuminum cyminum essential oils on planktonic and biofilm forms of Salmonella typhimurium isolates. 3 Biotech 10(7):1–10. https://doi.org/10.1007/s13205-020-02286-2
Tseng SP, Hung WC, Huang CY et al (2016) 5-Episinuleptolide decreases the expression of the extracellular matrix in early biofilm Formation of multi-drug resistant Acinetobacter baumannii. Mar drugs 14(8):143. https://doi.org/10.3390/md14080143
McConnell MJ, Pérez-Ordóñez A, Pérez-Romero P et al (2012) Quantitative real-time PCR for detection of Acinetobacter baumannii colonization in the hospital environment. J Clin Microbiol 50(4):1412–1414. https://doi.org/10.1128/JCM.06566-11
Pourhajibagher M, Boluki E, Chiniforush N et al (2016) Modulation of virulence in Acinetobacter baumannii cells surviving photodynamic treatment with toluidine blue. Photodiagnosis Photodyn Ther 15:202–212. https://doi.org/10.1016/j.pdpdt.2016.07.007
Azizi O, Shahcheraghi F, Salimizand H et al (2016) Molecular analysis and expression of bap gene in biofilm-forming multi-drug-resistant Acinetobacter baumannii. Rep Biochem Mol Biol 5(1):62–72
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Iluz N, Maor Y, Keller N et al (2018) The synergistic effect of PDT and oxacillin on clinical isolates of Staphylococcus aureus. Lasers Surg Med 50(5):535–551. https://doi.org/10.1002/lsm.22785
Ronqui MR, de Aguiar Coletti TM, de Freitas LM et al (2016) Synergistic antimicrobial effect of photodynamic therapy and ciprofloxacin. J Photochem Photobiol B 158:122–129. https://doi.org/10.1016/j.jphotobiol.2016.02.036
Chiniforush N, Pourhajibagher M, Shahabi S et al (2015) Clinical approach of high technology techniques for control and elimination of endodontic microbiota. J Lasers Med Sci 6(4):139–150. https://doi.org/10.15171/jlms.2015.09
Vera DMA, Haynes MH, Ball AR et al (2012) Strategies to potentiate antimicrobial photoinactivation by overcoming resistant phenotypes. Photochem Photobiol 88(3):499–511. https://doi.org/10.1111/j.1751-1097.2012.01087.x
De Melo WC, Avci P, de Oliveira MN et al (2013) Photodynamic inactivation of biofilm: taking a lightly colored approach to stubborn infection. Expert Rev Anti Infect Ther 11(7):669–693. https://doi.org/10.1586/14787210.2013.811861
Pourhajibagher M, Chiniforush N, Shahabi S et al (2016) Sub-lethal doses of photodynamic therapy affect biofilm formation ability and metabolic activity of Enterococcus faecalis. Photodiagnosis Photodyn Ther 15:159–166. https://doi.org/10.1016/j.pdpdt.2016.06.003
Dai T, Tegos GP, Lu Z et al (2009) Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother 53(9):3929–3934. https://doi.org/10.1128/AAC.00027-09
Cho K, Lee SY, Chang BS et al (2015) The effect of photodynamic therapy on Aggregatibacter actinomycetemcomitans attached to surface-modified titanium. J Periodontal Implant Sci 45(2):38–45. https://doi.org/10.5051/jpis.2015.45.2.38
Chow JY, Yang Y, Tay SB et al (2014) Disruption of biofilm formation by the human pathogen Acinetobacter baumannii using engineered quorum-quenching lactonases. Antimicrob Agents Chemother 58(3):1802–1805. https://doi.org/10.1128/AAC.02410-13
Wood S, Metcalf D, Devine D et al (2006) Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother 57(4):680–684. https://doi.org/10.1093/jac/dkl021
Tubby S, Wilson M, Nair SP (2009) Inactivation of staphylococcal virulence factors using a light-activated antimicrobial agent. BMC Microbiol 9(1):211. https://doi.org/10.1186/1471-2180-9-211
Issam AA, Zimmermann S, Reichling J et al (2015) Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 22(2):245–255. https://doi.org/10.1016/j.phymed.2014.11.019
Funding
This research did not receive any grant.
Author information
Authors and Affiliations
Contributions
LB: conceptualization, methodology, experimental works, statistical analysis, article writing; MG: methodology, experimental works, statistical analysis; MMR: experimental works. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The protocol was approved by the Ethics Committee of Islamic Azad Tehran Medical Sciences University (Code: IR.IAU.PS.REC.1399.197).
Consent to Participate
All subjects gave their informed consent for inclusion before they participated in the study and volunteers’ data were anonymized before analysis.
Consent for Publication
The participants have consented to the submission of the manuscript to the journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Babaeekhou, L., Ghane, M. & Mohammad Rafiee, M. Photodynamic Therapy and Its Synergism with Melittin Against Drug-Resistant Acinetobacter baumannii Isolates with High Biofilm Formation Ability. Curr Microbiol 80, 324 (2023). https://doi.org/10.1007/s00284-023-03356-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03356-3