Abstract
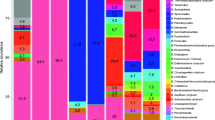
Sulfur-oxidizing bacteria, especially those from hot springs, have attracted the attention of microbiologists for more than 150 years. In contrast, the microbial diversity of cold sulfur springs remains largely unrecognized. Culture-dependent and culture-independent approaches were used to study the diversity of sulfur-oxidizing bacterial communities in two cold sulfur springs in Slovakia. Geological conditions and resulting spring water chemistry appear to be major factors influencing the composition of the sulfur-oxidizing bacterial community. Bacterial communities in both springs were found to be dominated by Proteobacteria with Epsilonproteobacteria being prevalent in the high-salinity Stankovany spring and Alpha- and Gammaproteobacteria in the low-salinity Jovsa spring. Limited overlap was found between culture-dependent and culture-independent approaches with multiple taxa of cultivated sulfur-oxidizing bacteria not being detected by the culture-independent metagenomics approach. Moreover, four cultivated bacterial isolates could represent novel taxa based on the low similarity of their 16S rRNA gene sequence (similarity lower than 98%) to sequences of known bacteria. Our study supports the current view that multiple approaches are required to assess the bacterial diversity in natural habitats and indicates that sulfur springs in Slovakia harbor unique, yet-undescribed microorganisms.


Similar content being viewed by others
Data Availability
All 16S rRNA sequences were deposited to Genbank under the accession numbers OP420118-OP420127. The metagenomic sequence reads are available on Sequence Read Archive database under the BioProject accession numbers PRJNA877066 and PRJNA877070.
Code Availability
Not applicable.
References
Sorokin DY, Kuenen JG (2004) Haloalkaliphilic sulfur-oxidizing bacteria in soda lakes. FEMS Microbiol Rev 29:685–702. https://doi.org/10.1016/j.femsre.2004.10.005
Meier D, Pjevac P, Bach W, Hourdez S, Girguis PR, Vidoudez C, Amann R, Meyerdierks A (2017) Niche partitioning of diverse sulfur-oxidizing bacteria at hydrothermal vents. ISME J 11:1545–1558. https://doi.org/10.1038/ismej.2017.37
Elshahed MS, Najar FZ, Aycock M, Qu C, Roe BA, Krumholz LR (2005) Metagenomic analysis of the microbial community at Zodletone Spring (Oklahoma): Insights into the genome of a member of the novel candidate division OD1. Appl Environ Microbiol 71:7598–7602. https://doi.org/10.1128/AEM.71.11.7598-7602.2005
Perreault NN, Adersen DT, Pollard WH, Greer CW, Whyte LG (2007) Characterization of the prokaryotic diversity in cold saline perennial springs of the Canadian High Arctic. Appl Environ Microbiol 73:1532–1543. https://doi.org/10.1128/AEM.01729-06
Meziti A, Nikouli E, Hatt JK, Konstantinidis KT, Kormas KA (2021) Time series metagenomic sampling of the Thermopyles, Greece, geothermal springs reveals stable microbial communities dominated by novel sulfur-oxidizing chemoautotrophs. Environ Microbiol 23:3710–3726. https://doi.org/10.1111/1462-2920.15373
Chaudhary A, Haack SK, Duris JW, Marsh TL (2009) Bacterial and archaeal phylogenetic diversity of a cold sulfur-rich spring on the shoreline of Lake Erie, Michigan. Appl Environ Microbiol 75:5025–5036. https://doi.org/10.1128/AEM.00112-09
Amin A, Ahmed I, Salam N, Kim BY, Singh D, Zhi XY, Xiao M, Li WJ (2017) Diversity and distribution of thermophilic bacteria in hot springs of Pakistan. Microb Ecol 74:116–127. https://doi.org/10.1007/s00248-017-0930-1
Nosalova L, Fecskeova LK, Piknova M, Bonova K, Pristas P (2023) Unique populations of sulfur-oxidizing bacteria in natural cold sulfur springs in Slovakia. Geomicrobiology J. https://doi.org/10.1080/01490451.2023.2167021
Hájková P, Jamrichová E, Šolcová A, Frodlová J, Petr L, Dítě D, Hájek M, Horsák M (2020) Can relict-rich communities be of an anthropogenic origin? Palaeoecological insight into conservation strategy for endangered Carpathian travertine fens. Quat Sci Rev 234:106241. https://doi.org/10.1016/j.quascirev.2020.106241
Song J, Oh HM, Cho JC (2009) Improved culturability of SAR11 strains in dilution-to-extinction culturing from the East Sea. West Pacific Ocean FEMS Microbiol Lett 295(2):141–147. https://doi.org/10.1111/j.1574-6968.2009.01623.x
Pospiech A, Neumann B (1995) A versatile quick-prep of genomic DNA from Gram-positive bacteria. Trends Genet 11:217–218. https://doi.org/10.1016/s0168-9525(00)89052-6
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703. https://doi.org/10.1128/jb.173.2.697-703.1991
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ (2008) Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol 74:2461–2470. https://doi.org/10.1128/AEM.02272-07
Walker AW, Martin JC, Scott P, Parkhill J, Flint HJ, Scott KP (2015) 16S rRNA gene-based profiling of the human infant gut microbiota is strongly influenced by sample processing and PCR primer choice. Microbiome 3:26. https://doi.org/10.1186/s40168-015-0087-4
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. https://doi.org/10.1128/AEM.01541-09
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner F (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. https://doi.org/10.1093/nar/gks1219
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. https://doi.org/10.7717/peerj.2584
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. https://doi.org/10.1128/AEM.00062-07
Kuraku S, Zmasek CM, Nishimura O, Katoh K (2013) aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res 41:W22–W28. https://doi.org/10.1093/nar/gkt389
Louca S, Parfrey LW, Doebeli M (2016) Decoupling function and taxonomy in the global ocean microbiome. Science 353:1272–1277. https://doi.org/10.1126/science.aaf4507
Wickham H (2016) ggplot2: Elegant graphics for data analysis. Springer-Verlag, New York
Petri R, Podgorosek L, Imhoff JF (2001) Phylogeny and distribution of the soxB gene among thiosulfate-oxidizing bacteria. FEMS Microbiol Lett 197:171–178. https://doi.org/10.1111/j.1574-6968.2001.tb10600.x
Dul’tseva NM, Turova TP, Spiridonova EM, Kolganova TV, Osipov GA, Gorlenko VM, (2006) Thiobacillus sajanensis sp. nov., a new obligately autotrophic sulfur-oxidizing bacterium isolated from Khoito-Gol hydrogen-sulfide springs. Buryatia Microbiol 75:582–592. https://doi.org/10.1134/S0026261706050080
Slyemi D, Moinier D, Brochier-Armanet C, Bonnefoy V, Johnson DB (2011) Characteristics of a phylogenetically ambiguous, arsenic-oxidizing Thiomonas sp., Thiomonas arsenitoxydans strain 3As(T) sp nov. Arch Microbiol 193:439–449. https://doi.org/10.1007/s00203-011-0684-y
Scott MK, Williams J, Porter CMB, Russel S, Harmer TL, Paul JH, Antonen KM, Bridges MK, Camper GJ, Campla CK (2018) Genomes of ubiquitous marine and hypersaline Hydrogenovibrio, Thiomicrorhabdus and Thiomicrospira spp. encode a diversity of mechanisms to sustain chemolithoautotrophy in heterogeneous environments. Environ Microbiol 20:2686–2708. https://doi.org/10.1111/1462-2920.14090
Piccini C, Conde D, Alonso C, Sommaruga R, Pernthaler J (2006) Blooms of single bacterial species in a Coastal Lagoon of the Southwestern Atlantic Ocean. Appl Environ Microbiol 72(10):6560–6568. https://doi.org/10.1128/AEM.01089-06
Jiang H, Dong H, Yu B, Liu X, Li Y, Ji S, Zhang CL (2007) Microbial response to salinity change in Lake Chaka, a hypersaline lake on Tibetan plateau. Environ Microbiol 9(10):2603–2621. https://doi.org/10.1111/j.1462-2920.2007.01377.x
Campbell BJ, Engel AS, Porter ML, Takai K (2006) The versatile ε-proteobacteria: key players in sulphidic habitats. Nat Rev Microbiol 4:458–468. https://doi.org/10.1038/nrmicro1414
Wright KE, Williamson C, Grasby SE, Spear JR, Templeton AS (2013) Metagenomic evidence for sulfur lithotrophy by Epsilonproteobacteria as the major energy source for primary productivity in a sub-aerial arctic glacial deposit. Borup Fiord Pass Front Microbiol 4:63. https://doi.org/10.3389/fmicb.2013.00063
Han Y, Perner M (2015) The globally widespread genus Sulfurimonas: versatile energy metabolisms and adaptations to redox clines. Front Microbiol 6:989. https://doi.org/10.3389/fmicb.2015.00989
Headd B, Engel AS (2014) Biogeographic congruency among bacterial communities from terrestrial sulfidic springs. Front Microbiol 5:473. https://doi.org/10.3389/fmicb.2014.00473
Gulecal-Pektas Y, Temel M (2016) A window to the subsurface: microbial diversity in hot springs of a Sulfidic cave (Kaklik, Turkey). Geomicrobiology J 34(4):374–384. https://doi.org/10.1080/01490451.2016.1204374
Berg JS, Jézéquel D, Duverger A, Lamy D, Laberty-Robert C, Miot J (2019) Microbial diversity involved in iron and cryptic sulfur cycling in the ferruginous, low-sulfate waters of Lake Pavin. PLoS ONE 14(2):e0212787. https://doi.org/10.1371/journal.pone.0212787
Valeriani F, Crognale S, Protano C, Gianfranceschi G, Orsini M, Vitali M, Spica VR (2018) Metagenomic analysis of bacterial community in a travertine depositing hot spring. New Microbiol 41(2):126–135
Gandham S, Lodha T, Chintalapati S, Chintalapati VR (2018) Rhodobacter alkalitolerans sp. nov., isolated from an alkaline brown pond. Arch Microbiol 200:1487–1492. https://doi.org/10.1007/s00203-018-1561-8
Satagopan S, Huening KA, Tabita FR (2019) Selection of Cyanobacterial (Synechococcus sp. strain PCC 6301) RubisCO variants with improved functional properties that confer enhanced CO2-dependent growth of Rhodobacter capsulatus, a photosynthetic bacterium. MBio 10(4):e01537-e1619. https://doi.org/10.1128/mBio.01537-19
Ghosh W, Dam B (2009) Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea. FEMS Microbiol Rev 33:999–1043. https://doi.org/10.1111/j.1574-6976.2009.00187.x
Abraham WR, Rohde M, Bennasar A (2014) The Family Caulobacteraceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The Prokaryotes. Springer, Heidelberg, pp 179–205
Zhang RC, Chen C, Wang W, Shao B, Xu XJ, Zhou X, Lee DJ, Ren NQ (2020) The stimulating metabolic mechanisms response to sulfide and oxygen in typical heterotrophic sulfide-oxidizing nitrate-reducing bacteria Pseudomonas C27. Bioresour Technol 309:123451. https://doi.org/10.1016/j.biortech.2020.123451
Masuda S, Eda S, Ikeda S, Mitsui H, Minamisawa K (2010) Thiosulfate-dependent chemolithoautotrophic growth of Bradyrhizobium japonicum. Appl Environ Microbiol 76:2402–2409. https://doi.org/10.1128/AEM.02783-09
Aguilar JRP, Cabriales JJP, Vega MM (2008) Identification and characterization of sulfur-oxidizing bacteria in an artificial wetland that treats wastewater from a tannery. Int J Phytoremediation 10:359–370. https://doi.org/10.1080/15226510802100390
Moissl C, Rudolph C, Huber R (2002) Natural communities of novel archaea and bacteria with a string-of-pearls-likemorphology: molecular analysis of the bacterial partners. Appl Environ Microbiol 68:933–937. https://doi.org/10.1128/AEM.68.2.933-937.2002
Miseta R, Palatinszky M, Makk J, Márialigeti K, Borsodi AK (2012) Phylogenetic diversity of bacterial communities associated with sulfurous karstic well waters of a Hungarian spa. Geomicrobiol J 29:101–113. https://doi.org/10.1080/01490451.2011.558563
Funding
This work was supported by Pavol Jozef Safarik University Grant No. VVGS-PF-2021-1745.
Author information
Authors and Affiliations
Contributions
PP and LKF conceived and designed the analysis. LN collected the data. PP, JK, LN contributed to materials, data or analysis tools. LKF, JK, and LN performed the analyses. JK and LN interpreted the results. LN wrote the paper. MP, PP, and LN edited the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest with respect to the work performed in the manuscript.
Ethical Approval
Not applicable.
Consent to Participate
No applicable.
Consent for Publication
No applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nosalova, L., Kiskova, J., Fecskeova, L.K. et al. Bacterial Community Structure of Two Cold Sulfur Springs in Slovakia (Central Europe). Curr Microbiol 80, 145 (2023). https://doi.org/10.1007/s00284-023-03251-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03251-x