Abstract
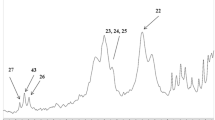
This study investigated the bacterial and postbiotic potential of three Anatolian bee bread samples obtained from different regions of Turkey (Marmara, Aegean, and Mediterranean) and offered for human consumption. The families most commonly found in Anatolian bee bread were Lactobacillaceae, Oscillospiraceae, Bacteroidaceae, Prevotellaceae, and Lachnospiraceae. Lactobacillus delbruckeii was highly abundant, but also other beneficial bacteria, known to be next-generation probiotics, were revealed in bee bread, such as Prevotalla copri, Faecalibacterium prausnitzii, and Akkermansia muciniphila. Apart from these beneficial bacteria, bee bread samples also harbored undesired bacteria such as Phocaeicola vulgatus, Phocaeicola dorei, and Clostridium perfringens. Fatty acid composition showed that bee bread samples had butyric acid, a short-chain fatty acid, as a postbiotic. Additionally, polyunsaturated fatty acids were also found such as alfa-linolenic acid and eicosadienoic acid. The fatty acids with the highest amounts were palmitic acid (~ 30%), stearic acid (~ 17%), and alpha-linolenic acid (~ 12%). One of the samples exhibited antimicrobial activity against Staphylococcus aureus.




Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Kafantaris I, Amoutzias GD, Mossialos D (2021) Foodomics in bee product research: a systematic literature review. Eur Food Res Technol 247:309–331. https://doi.org/10.1007/s00217-020-03634-5
Jaya F, Rosyidi D, Radiati LE et al (2020) Antioxidant activity and microbiological quality of bee bread collected from three different species honey bee. IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/475/1/012033
Barta DG, Cornea-cipcigan M, Margaoan R (2022) Biotechnological processes simulating the natural fermentation process of bee bread and therapeutic properties an overview. Front Nutr. https://doi.org/10.3389/fnut.2022.871896
Zuluaga-Dominguez CM, Fuenmayor CA (2022) Bee bread and gut microbiota. In: Dilek Boyacıoğlu (ed) Bee Products and Their Applications in the Food and Pharmaceutical Industries. Elsevier Inc., 315–345
Mohammad SM, Mahmud-Ab-Rashid NK, Zawawi N (2021) Stingless bee-collected pollen (bee bread): chemical and microbiology properties and health benefits. Molecules 26:1–29. https://doi.org/10.3390/molecules26040957
Dranca F, Ursachi F, Oroian M (2020) Bee bread: Physicochemical characterization and phenolic content extraction optimization. Foods. https://doi.org/10.3390/foods9101358
Didaras NA, Karatasou K, Dimitriou TG et al (2020) Antimicrobial activity of bee-collected pollen and beebread: State of the art and future perspectives. Antibiotics 9:1–29. https://doi.org/10.3390/antibiotics9110811
Vásquez A, Olofsson TC (2009) The lactic acid bacteria involved in the production of bee pollen and bee bread. J Apic Res 48:189–195. https://doi.org/10.3896/IBRA.1.48.3.07
Putri SP, Ikram MMM, Sato A et al (2022) Application of gas chromatography-mass spectrometry-based metabolomics in food science and technology. J Biosci Bioeng 133:425–435. https://doi.org/10.1016/j.jbiosc.2022.01.011
Gao Y, Hou L, Gao J et al (2021) Metabolomics approaches for the comprehensive evaluation of fermented foods: a review. Foods 10:1–18. https://doi.org/10.3390/foods10102294
Kahraman-Ilıkkan Ö, Bağdat EŞ (2022) Metataxonomic sequencing to assess microbial safety of Turkish white cheeses. Brazilian J Microbiol. https://doi.org/10.1007/s42770-022-00730-4
Ilıkkan ÖK, Bağdat EŞ (2021) Comparison of bacterial and fungal biodiversity of Turkish kefir grains with high-throughput metagenomic analysis. Lwt. https://doi.org/10.1016/j.lwt.2021.112375
ISO7889:2003 (2003) Yogurt — Enumeration of characteristic microorganisms. Int Organ Stand
Pełka K, Otłowska O, Worobo RW, Szweda P (2021) Bee bread exhibits higher antimicrobial potential compared to bee pollen. Antibiotics 10:1–14. https://doi.org/10.3390/antibiotics10020125
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Singh NK, Wood JM, Karouia F, Venkateswaran K (2018) Succession and persistence of microbial communities and antimicrobial resistance genes associated with International Space Station environmental surfaces. Microbiome 6(1):1–23
BioBam Bioinformatics (2022) OmicsBox-Bioinformatics Made Easy Available
Sánchez MC, Valdés A, Velapatiño A et al (2022) Metataxonomic and metabolomic evidence of biofilm homeostasis disruption related to caries: an in vitro study. Mol Oral Microbiol 37(2):81–96. https://doi.org/10.1111/omi.12363
Phillips CD, Phelan G, Dowd SE et al (2012) Microbiome analysis among bats describes influences of host phylogeny, life history, physiology and geography. Mol Ecol 21:2617–2627. https://doi.org/10.1111/j.1365-294X.2012.05568.x
Hammer Ø, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Engel P, Kwong WK, Moran NA (2013) Frischella perrara gen. nov., sp. nov., a gammaproteobacterium isolated from the gut of the honeybee, Apis mellifera. Int J Syst Evol Microbiol 63:3646–3651. https://doi.org/10.1099/ijs.0.049569-0
Tully JG, Whitcomb RF, Hackett KJ et al (1998) Entornoplasma freundtii sp. nov., a new species from a green tiger beetle (Coleoptera: Cicindel idae). Int J Syst Bacteriol 48(4):1197–1204
Keller A, Brandel A, Becker MC et al (2018) Wild bees and their nests host Paenibacillus bacteria with functional potential of avail. Microbiome 6:1–10. https://doi.org/10.1186/s40168-018-0614-1
Mărgăoan R, Stranț M, Varadi A et al (2019) Bee collected pollen and bee bread: bioactive constituents and health benefits. Antioxidants 8:1–33. https://doi.org/10.3390/antiox8120568
Silva YP, Bernardi A, Frozza RL (2020) The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 11:1–14. https://doi.org/10.3389/fendo.2020.00025
Riwes M, Reddy P (2020) Short chain fatty acids: postbiotics/metabolites and graft versus host disease colitis. Semin Hematol 57:1–6. https://doi.org/10.1053/j.seminhematol.2020.06.001
Kaplan M, Karaoglu Ö, Silici S (2019) An evaluation on bee bread: chemical and palynological analysis. Mellifera 19:21–29
Kaplan M, Karaoglu Ö, Eroglu N, Silici S (2016) Fatty acid and proximate composition of bee bread. Food Technol Biotechnol 54:497–504
Bakour M, Fernandes Â, Barros L et al (2019) Bee bread as a functional product: chemical composition and bioactive properties. Lwt 109:276–282. https://doi.org/10.1016/j.lwt.2019.02.008
Human H, Nicolson SW (2006) Nutritional content of fresh, bee-collected and stored pollen of aloe greatheadii var. davyana (Asphodelaceae). Phytochemistry 67:1486–1492. https://doi.org/10.1016/j.phytochem.2006.05.023
Anderson KE, Carroll MJ, Sheehan T et al (2014) Hive-stored pollen of honey bees: many lines of evidence are consistent with pollen preservation, not nutrient conversion. Mol Ecol 23:5904–5917. https://doi.org/10.1111/mec.12966
Abouda Z, Zerdani I, Kalalou I et al (2011) The antibacterial activity of moroccan bee bread and bee-pollen (fresh and dried) against pathogenic bacteria. Res J Microbiol 6:376–384
Urcan A, Criste A, Dezmirean D et al (2018) Antimicrobial activity of bee bread extracts against different bacterial strains. Bull Univ Agric Sci Vet Med Cluj-Napoca Anim Sci Biotechnol 75(2):85. https://doi.org/10.15835/buasvmcn-asb:2018.0004
Muñoz-Colmenero M, Baroja-Careaga I, Kovačić M et al (2020) Differences in honey bee bacterial diversity and composition in agricultural and pristine environments – a field study. Apidologie 51:1018–1037. https://doi.org/10.1007/s13592-020-00779-w
Disayathanoowat T, Li H, Supapimon N et al (2020) Different dynamics of bacterial and fungal communities in hive-stored bee bread and their possible roles: A case study from two commercial honey bees in china. Microorganisms. https://doi.org/10.3390/microorganisms8020264
Bakour M, Laaroussi H, Ousaaid D et al (2022) Bee bread as a promising source of bioactive molecules and functional properties: an up-to-date review. Antibiotics 11:1–39. https://doi.org/10.3390/antibiotics11020203
Iorizzo M, Pannella G, Lombardi SJ et al (2020) Inter-and intra-species diversity of lactic acid bacteria in apis mellifera ligustica colonies. Microorganisms 8:1–17. https://doi.org/10.3390/microorganisms8101578
Huang F, Sardari RRR, Jasilionis A et al (2021) Cultivation of the gut bacterium Prevotella copri DSM 18205T using glucose and xylose as carbon sources. Microbiologyopen 10:1–15. https://doi.org/10.1002/mbo3.1213
Khan MT, Duncan SH, Stams AJM et al (2012) The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic-anoxic interphases. ISME J 6:1578–1585. https://doi.org/10.1038/ismej.2012.5
Maioli TU, Borras-Nogues E, Torres L et al (2021) Possible benefits of faecalibacterium prausnitzii for obesity-associated gut disorders. Front Pharmacol 12:1–13. https://doi.org/10.3389/fphar.2021.740636
Cani PD, Depommier C, Derrien M et al (2022) Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol. https://doi.org/10.1038/s41575-022-00631-9
Cobo F, Peerez-Carrasco V, Rodríguez-Guerrero E et al (2022) Misidentification of Phocaeicola (Bacteroides) dorei in two patients with bacteremia. Anaerobe 75:6–8. https://doi.org/10.1016/j.anaerobe.2022.102544
Acknowledgements
The author would like to thank Isparta University of Applied Sciences, Research Center for the FAME analysis. This study was performed in Başkent University Food Processing Laboratory.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
OKI: design of experiment, analyses, and interpretation of all experiments, writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Ethical approval is not applicable, because this article does not contain any studies with human or animal subjects.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kahraman-Ilıkkan, Ö. Bacterial Profile and Fatty Acid Composition of Anatolian Bee Bread Samples by Metataxonomic and Metabolomic Approach. Curr Microbiol 80, 90 (2023). https://doi.org/10.1007/s00284-023-03195-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03195-2