Abstract
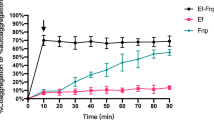
The type II toxin-antitoxin (T-A) HicAB system is abundant in several bacteria and archaea, such as Escherichia coli, Burkholderia Pseudomallei, Yersinia pestis, Pseudomonas aeruginosa, and Streptococcus pneumoniae. This system engages in stress response, virulence, and bacterial persistence. This study showed that the biofilm-forming ability of the hicAB deletion mutant was significantly decreased to moderate ability compared to the extra-intestinal pathogenic Escherichia coli (ExPEC) parent strain and the complemented strain, which are strong biofilm producers. Congo red assay showed that the hicAB mutant maintained the ability to form curli fimbriae. Using RNA-seq and comparative real-time quantitative RT-PCR, we observed the difference in gene expression between the hicAB mutant and the parent strain, which was associated with biofilm formation. Our data indicate that the HicAB type II T-A system has a key role in biofilm formation by ExPEC, which may be associated with outer membrane protein (OMP) gene expression. Collectively, our results indicate that the hicAB type II T-A system is involved in ExPEC biofilm formation.




Similar content being viewed by others
Data Availability
The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (BioSample Accession Number: SAMN31830681, SAMN31830682; BioProject PRJNA904110).
References
Ogura T, Hiraga S (1983) Mini-F plasmid genes that couple host cell division to plasmid proliferation. Proc Natl Acad Sci USA 80(15):4784–4788
Yamaguchi Y, Park JH, Inouye M (2011) Toxin-antitoxin systems in bacteria and archaea. Annu Rev Genet 45:61–79
Singh G, Yadav M, Ghosh C, Rathore JS (2021) Bacterial toxin-antitoxin modules: classification, functions, and association with persistence. Curr Res Microb Sci 2:100047. https://doi.org/10.1016/j.crmicr.2021.100047
Pandey DP, Gerdes K (2005) Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res 33(3):966–976
Jurenas D, Fraikin N, Goormaghtigh F, Van Melderen L (2022) Biology and evolution of bacterial toxin-antitoxin systems. Nat Rev Microbiol 20(6):335–350
Leroux M, Laub MT (2022) Toxin-Antitoxin systems as phage defense elements. Annu Rev Microbiol 76:21–43
Al Marjani MF, Authman SH, Ali FS (2020) Toxin–antitoxin systems and biofilm formation in bacteria. Rev Med Microbiol 31(2):61–69
Kamruzzaman M, Wu AY, Iredell JR (2021) Biological functions of type II toxin-antitoxin systems in bacteria. Microorganisms. https://doi.org/10.3390/microorganisms9061276
Wen Y, Behiels E, Devreese B (2014) Toxin-antitoxin systems: their role in persistence, biofilm formation, and pathogenicity. Pathog Dis 70(3):240–249
Zhao J, Wang Q, Li M, Heijstra BD, Wang S, Liang Q, Qi Q (2013) Escherichia coli toxin gene hipA affects biofilm formation and DNA release. Microbiology 159(3):633–640
Soo VWC, Wood TK (2013) Antitoxin MqsA represses curli formation through the master biofilm regulator CsgD. Sci Rep-Uk. https://doi.org/10.1038/srep03186
Ma D, Gu H, Shi Y, Huang H, Sun D, Hu Y (2021) Edwardsiella piscicida YefM-YoeB: a type II toxin-antitoxin system that is related to antibiotic resistance, biofilm formation, serum survival, and host infection. Front Microbiol. https://doi.org/10.3389/fmicb.2021.646299
Chirwa NT, Herrington MB (2003) CsgD, a regulator of curli and cellulose synthesis, also regulates serine hydroxymethyltransferase synthesis in Escherichia coli K-12. Microbiology 149(2):525–535
Mhlanga-Mutangadura T, Morlin G, Smith AL, Eisenstark A, Golomb M (1998) Evolution of the major pilus gene cluster of Haemophilus influenzae. J Bacteriol 180(17):4693–4703
Jorgensen MG, Pandey DP, Jaskolska M, Gerdes K (2009) HicA of Escherichia coli defines a novel family of translation-independent mRNA interferases in bacteria and archaea. J Bacteriol 191(4):1191–1199
Butt A, Higman VA, Williams C, Crump MP, Hemsley CM, Harmer N, Titball RW (2014) The HicA toxin from Burkholderia pseudomallei has a role in persister cell formation. Biochem J 459(2):333–344
Bibi-Triki S, Li DLSI, Lazar N, Leroy A, Van Tilbeurgh H, Sebbane F, Pradel E (2014) Functional and structural analysis of HicA3-HicB3, a novel toxin-antitoxin system of Yersinia pestis. J Bacteriol 196(21):3712–3723
Kim DH, Kang SM, Park SJ, Jin C, Yoon HJ, Lee BJ (2018) Functional insights into the Streptococcus pneumoniae HicBA toxin-antitoxin system based on a structural study. Nucleic Acids Res 46(12):6371–6386
Makarova KS, Grishin NV, Koonin EV (2006) The HicAB cassette, a putative novel, RNA-targeting toxin-antitoxin system in archaea and bacteria. Bioinformatics 22(21):2581–2584
Li G, Shen M, Lu S, Le S, Tan Y, Wang J, Zhao X, Shen W, Guo K, Yang Y, Zhu H, Rao X, Hu F, Li M (2016) Identification and characterization of the HicAB toxin-antitoxin system in the opportunistic pathogen Pseudomonas aeruginosa. Toxins (Basel) 8(4):113. https://doi.org/10.3390/toxins8040113
Potnis AA, Raghavan PS, Shelke A, Nikam TD, Rajaram H (2017) Comparative analysis of MazEF and HicAB toxin-antitoxin systems of the cyanobacterium, Anabaena sp. PCC7120. Fems Microbiol Lett. https://doi.org/10.1093/femsle/fnw279
Thomet M, Trautwetter A, Ermel G, Blanco C (2019) Characterization of HicAB toxin-antitoxin module of Sinorhizobium meliloti. Bmc Microbiol 19(1):10. https://doi.org/10.1186/s12866-018-1382-6
Maisonneuve E, Shakespeare LJ, Jorgensen MG, Gerdes K (2011) Bacterial persistence by RNA endonucleases. Proc Natl Acad Sci USA 108(32):13206–13211
Daimon Y, Narita S, Akiyama Y (2015) Activation of toxin-antitoxin system toxins suppresses lethality caused by the loss of σE in Escherichia coli. J Bacteriol 197(14):2316–2324
Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ (1987) Bacterial biofilms in nature and disease. Annu Rev Microbiol 41:435–464
Janssens JC, Steenackers H, Robijns S, Gellens E, Levin J, Zhao H, Hermans K, De Coster D, Verhoeven TL, Marchal K, Vanderleyden J, De Vos DE, De Keersmaecker SC (2008) Brominated furanones inhibit biofilm formation by Salmonella enterica serovar Typhimurium. Appl Environ Microbiol 74(21):6639–6648
Beloin C, Roux A, Ghigo JM (2008) Escherichia coli biofilms. Curr Top Microbiol Immunol 322:249–289
Srey S, Jahid IK, Ha S (2013) Biofilm formation in food industries: a food safety concern. Food Control 31(2):572–585
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284(5418):1318–1322
Anderson GG, O’Toole GA (2008) Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol 322:85–105
Dobrindt U, Hacker J (2008) Targeting virulence traits: Potential strategies to combat extraintestinal pathogenic E. coli infections. Curr Opin Microbiol 11(5):409–413
Wasinski B (2019) Extra-intestinal pathogenic Escherichia coli -threat connected with food-borne infections. Ann Agric Environ Med 26(4):532–537
Jakobsen L, Spangholm DJ, Pedersen K, Jensen LB, Emborg HD, Agerso Y, Aarestrup FM, Hammerum AM, Frimodt-Moller N (2010) Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. Int J Food Microbiol 142(1–2):264–272
Xia X, Meng J, Zhao S, Bodeis-Jones S, Gaines SA, Ayers SL, Mcdermott PF (2011) Identification and antimicrobial resistance of extraintestinal pathogenic Escherichia coli from retail meats. J Food Prot 74(1):38–44
Massella E, Giacometti F, Bonilauri P, Reid CJ, Djordjevic SP, Merialdi G, Bacci C, Fiorentini L, Massi P, Bardasi L, Rubini S, Savini F, Serraino A, Piva S (2021) Antimicrobial resistance profile and ExPEC virulence potential in commensal Escherichia coli of multiple sources. Antibiotics (Basel). https://doi.org/10.3390/antibiotics10040351
Osugui L, de Castro AF, Iovine R, Irino K, Carvalho VM (2014) Virulence genotypes, antibiotic resistance and the phylogenetic background of extraintestinal pathogenic Escherichia coli isolated from urinary tract infections of dogs and cats in Brazil. Vet Microbiol 171(1–2):242–247
Platell JL, Johnson JR, Cobbold RN, Trott DJ (2011) Multidrug-resistant extraintestinal pathogenic Escherichia coli of sequence type ST131 in animals and foods. Vet Microbiol 153(1–2):99–108
Meng X, Zhang L, Hou B, Liu X, Li S (2016) Oxygen-Free condition inhibited biofilm formation in extraintestinal pathogenic Escherichia coli strain PPECC42 through preventing curli production. Curr Microbiol 73(2):153–158
Hou B, Meng XR, Zhang LY, Tan C, Jin H, Zhou R, Gao JF, Wu B, Li ZL, Liu M, Chen HC, Bi DR, Li SW (2014) TolC promotes ExPEC biofilm formation and curli production in response to medium osmolarity. Biomed Res Int. https://doi.org/10.1155/2014/574274
Li B, Huang Q, Cui A, Liu X, Hou B, Zhang L, Liu M, Meng X, Li S (2018) Overexpression of outer membrane protein X (OmpX) compensates for the effect of TolC inactivation on biofilm formation and curli production in extraintestinal pathogenic Escherichia coli (ExPEC). Front Cell Infect Microbiol 8:208. https://doi.org/10.3389/fcimb.2018.00208
Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M (2000) A modified microtiter-plate test for quantification of Staphylococcal biofilm formation. J Microbiol Methods 40(2):175–179
Bokranz W, Wang X, Tschape H, Romling U (2005) Expression of cellulose and curli fimbriae by Escherichia coli isolated from the gastrointestinal tract. J Med Microbiol 54(Pt 12):1171–1182
Zogaj X, Bokranz W, Nimtz M, Romling U (2003) Production of cellulose and curli fimbriae by members of the family Enterobacteriaceae isolated from the human gastrointestinal tract. Infect Immun 71(7):4151–4158
Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, Feng T, Zhou L, Tang W, Zhan L, Fu X, Liu S, Bo X, Yu G (2021) ClusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation (NY) 2(3):100141. https://doi.org/10.1016/j.xinn.2021.100141
Yu G, Wang LG, Han Y, He QY (2012) ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16(5):284–287
Lee JH, Kim YG, Cho MH, Wood TK, Lee J (2011) Transcriptomic analysis for genetic mechanisms of the factors related to biofilm formation in Escherichia coli O157:H7. Curr Microbiol 62(4):1321–1330
Goeders N, Van Melderen L (2014) Toxin-antitoxin systems as multilevel interaction systems. Toxins (Basel) 6(1):304–324
Barnhart MM, Chapman MR (2006) Curli biogenesis and function. Annu Rev Microbiol 60:131–147
Ma Q, Wood TK (2009) OmpA influences Escherichia coli biofilm formation by repressing cellulose production through the CpxRA two-component system. Environ Microbiol 11(10):2735–2746
Yoo BK, Chen J (2009) Influence of culture conditions and medium composition on the production of cellulose by shiga toxin-producing Escherichia coli cells. Appl Environ Microbiol 75(13):4630–4632
Zgurskaya HI, Krishnamoorthy G, Ntreh A, Lu S (2011) Mechanism and function of the outer membrane channel TolC in multidrug resistance and physiology of enterobacteria. Front Microbiol 2:189. https://doi.org/10.3389/fmicb.2011.00189
Ritter A, Com E, Bazire A, Goncalves MS, Delage L, Le Pennec G, Pineau C, Dreanno C, Compere C, Dufour A (2012) Proteomic studies highlight outer-membrane proteins related to biofilm development in the marine bacterium Pseudoalteromonas sp. D41. Proteomics 12(21):3180–3192
Cattelan N, Villalba MI, Parisi G, Arnal L, Serra DO, Aguilar M, Yantorno O (2016) Outer membrane protein OmpQ of Bordetella bronchiseptica is required for mature biofilm formation. Microbiol (Soc Gen Microbiol) 162(2):351
Garavaglia M, Rossi E, Landini P (2012) The pyrimidine nucleotide biosynthetic pathway modulates production of biofilm determinants in Escherichia coli. PLoS ONE 7(2):e31252. https://doi.org/10.1371/journal.pone.0031252
Acknowledgements
The authors declare they have no acknowledgements.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number 31802190 to B Hou) and the Natural Science Foundation of Fujian Province (Grant Number 2018J05053 to B Hou).
Author information
Authors and Affiliations
Contributions
BH and SWL conceived and designed the research. BH did the bioinformatics analyses and wrote the manuscript. CYW, YLC, and QYC helped conducting the experiments. LJZ helped designing the study.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publication
Not applicable
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, B., Wang, CY., Li, SW. et al. Effects of Toxin-Antitoxin System HicAB on Biofilm Formation by Extraintestinal Pathogenic E. coli. Curr Microbiol 80, 50 (2023). https://doi.org/10.1007/s00284-022-03138-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03138-3