Abstract
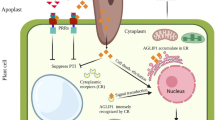
Necrosis and Ethylene-inducing peptide 1-like proteins (NLPs) are broadly distributed across bacteria, fungi, and oomycetes. Cytotoxic NLPs are usually secreted into the host apoplast where they can induce cell death and trigger plant immune responses in eudicots. To investigate the evolutionary history of the NLPs, we accessed the genomic resources of 79 species from 15 orders of Dothideomycetes. Phylogenetic approaches searched for biased patterns of NLP gene evolution and aimed to provide a phylogenetic framework for the cytotoxic activities of NLPs. Among Dothideomycetes, the NLP superfamily sizes varied, but usually contained from one to six members. Superfamily sizes were higher among pathogenic fungi, with family members that were mostly putative-effector NLPs. Across species, members of the NLP1 family (Type I NLPs) were predominant (84%) over members of the NLP2 family (Type II NLPs). The NLP1 family split into two subfamilies (NLP1.1 and NLP1.2). The NLP1.1 subfamily was broadly distributed across Dothideomycetes. There was strong agreement between the phylogenomics of Dothideomycetes and the phylogenetic tree based on members of the NLP1 subfamilies. To a lesser extent, phylogenomics also agreed with the phylogeny based on members of the NLP2 family. While gene losses seem to have shaped the evolutionary history of NLP2 family, ancient gene duplications followed by descent with modification characterized the NLP1 family. The strongest cytotoxic activities were recorded on NLPs of the NLP1.1 subfamily, suggesting that biased NLP gene retention in this subfamily favored the cytotoxic paralogs.





Similar content being viewed by others
Data Availability
This study used genomic data available from public repositories and their information is in the Electronic Supplementary Material.
Code Availability
Not applicable.
References
Gijzen M, Nürnberger T (2006) Nep1-like proteins from plant pathogens: recruitment and diversification of the NPP1 domain across taxa. Phytochemistry 67:1800–1807. https://doi.org/10.1016/j.phytochem.2005.12.008
Pemberton CL, Salmond GPC (2004) The Nep1-like proteins—a growing family of microbial elicitors of plant necrosis. Mol Plant Pathol 5:353–359. https://doi.org/10.1111/j.1364-3703.2004.00235.x
Seidl MF, Van den Ackerveken G (2019) Activity and phylogenetics of the broadly occurring family of microbial Nep1-Like Proteins. Annu Rev Phytopathol 57:367–386. https://doi.org/10.1146/annurev-phyto-082718-100054
Bailey BA (1995) Purification of a protein from culture filtrates of Fusarium oxysporum that induces ethylene and necrosis in leaves of Erythroxylum coca. Phytopathology 85:1035–1039
Levin E, Raphael G, Ma J et al (2019) Identification and functional analysis of NLP-encoding genes from the postharvest pathogen Penicillium expansum. Microorganisms. https://doi.org/10.3390/microorganisms7060175
Amsellem Z, Cohen BA, Gressel J (2002) Engineering hypervirulence in a mycoherbicidal fungus for efficient weed control. Nat Biotechnol 20:1035–1039. https://doi.org/10.1038/nbt743
Fang YL, Peng YL, Fan J (2017) The Nep1-like protein family of Magnaporthe oryzae is dispensable for the infection of rice plants. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-04430-0
Staats M, van Baarlen P, Schouten A et al (2007) Positive selection in phytotoxic protein-encoding genes of Botrytis species. Fungal Genet Biol 44:52–63. https://doi.org/10.1016/j.fgb.2006.07.003
Cobos R, Calvo-Peña C, Álvarez-Pérez JM et al (2019) Necrotic and cytolytic activity on grapevine leaves produced by Nep1-like proteins of Diplodia seriata. Front Plant Sci 10:1–13. https://doi.org/10.3389/fpls.2019.01282
Pour FN, Cobos R, Coque JJR et al (2020) Toxicity of recombinant Necrosis and Ethylene-Inducing Proteins (NLPs) from Neofusicoccum parvum. Toxins 12:1–17. https://doi.org/10.3390/toxins12040235
Böhm H, Albert I, Oome S et al (2014) A conserved peptide pattern from a widespread microbial virulence factor triggers pattern-induced immunity in Arabidopsis. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1004491
Cabral A, Oome S, Sander N et al (2012) Nontoxic Nep1-like proteins of the downy mildew pathogen Hyaloperonospora arabidopsidis: Repression of necrosis-inducing activity by a surface-exposed region. Mol Plant-Microbe Interact 25:697–708. https://doi.org/10.1094/MPMI-10-11-0269
Oome S, Raaymakers TM, Cabral A et al (2014) Nep1-like proteins from three kingdoms of life act as a microbe-associated molecular pattern in Arabidopsis. Proc Natl Acad Sci USA 111:16955–16960. https://doi.org/10.1073/pnas.1410031111
Qutob D, Kemmerling B, Brunner F et al (2006) Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell 18:3721–3744. https://doi.org/10.1105/tpc.106.044180
Fellbrich G, Romanski A, Varet A et al (2002) NPP1, a Phytophthora-associated trigger of plant defense in parsley and Arabidopsis. Plant J 32:375–390. https://doi.org/10.1046/j.1365-313X.2002.01454.x
Ottmann C, Luberacki B, Küfner I et al (2009) A common toxin fold mediates microbial attack and plant defense. Proc Natl Acad Sci USA 106:10359–10364. https://doi.org/10.1073/pnas.0902362106
Oome S, Van Den Ackerveken G (2014) Comparative and functional analysis of the widely occurring family of Nep1-like proteins. Mol Plant-Microbe Interact 27:1081–1094. https://doi.org/10.1094/MPMI-04-14-0118-R
Hyde KD, Jones EBG, Liu JK et al (2013) Families of Dothideomycetes. Fungal Divers 63:1–313. https://doi.org/10.1007/s13225-013-0263-4
Kirk P, Cannon P, Minter D, Stalpers J (2008) Ainsworth and Bisby’s dictionary of the Fungi, 10th edn. CAB International, Wallingford
Haridas S, Albert R, Binder M et al (2020) 101 Dothideomycetes genomes: a test case for predicting lifestyles and emergence of pathogens. Stud Mycol 96:141–153. https://doi.org/10.1016/j.simyco.2020.01.003
Schoch CL, Crous PW, Groenewald JZ et al (2009) A class-wide phylogenetic assessment of Dothideomycetes. Stud Mycol 64:1–15. https://doi.org/10.3114/sim.2009.64.01
Liu JK, Hyde KD, Jeewon R et al (2017) Ranking higher taxa using divergence times: a case study in Dothideomycetes. Fungal Divers 84:75–99. https://doi.org/10.1007/s13225-017-0385-1
Ohm RA, Feau N, Henrissat B et al (2012) Diverse lifestyles and strategies of plant pathogenesis encoded in the genomes of eighteen Dothideomycetes fungi. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1003037
Grigoriev IV, Nikitin R, Haridas S et al (2013) MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Res 42:D699. https://doi.org/10.1093/nar/gkt1183
Nguyen NH, Song Z, Bates ST et al (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248. https://doi.org/10.1016/j.funeco.2015.06.006
Emms DM, Kelly S (2015) OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol 16:1–14. https://doi.org/10.1186/s13059-015-0721-2
Buchfink B, Xie C, Huson DH (2014) Fast and sensitive protein alignment using DIAMOND. Nat Methods 12:59–60. https://doi.org/10.1038/nmeth.3176
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. https://doi.org/10.1093/bioinformatics/btp348
Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. https://doi.org/10.1093/molbev/msu300
El-Gebali S, Mistry J, Bateman A et al (2019) The Pfam protein families database in 2019. Nucleic Acids Res 47:D427–D432. https://doi.org/10.1093/nar/gky995
Jones P, Binns D, Chang HY et al (2014) InterProScan 5: genome-scale protein function classification. Bioinformatics 30:1236–1240. https://doi.org/10.1093/bioinformatics/btu031
Petersen TN, Brunak S, Von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. https://doi.org/10.1038/nmeth.1701
Krogh A, Larsson B, Von Heijne G, Sonnhammer ELL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. https://doi.org/10.1006/jmbi.2000.4315
Emanuelsson O, Brunak S, von Heijne G, Nielsen H (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc 2:953–971. https://doi.org/10.1038/nprot.2007.131
Li Y, Han Y, Qu M et al (2020) Apoplastic cell death-inducing proteins of filamentous plant pathogens: roles in plant-pathogen interactions. Front Genet. https://doi.org/10.3389/fgene.2020.00661
Santhanam P, Van Esse HP, Albert I et al (2013) Evidence for functional diversification within a fungal Nep1-like protein family. Mol Plant-Microbe Interact 26:278–286. https://doi.org/10.1094/MPMI-09-12-0222-R
Seidl MF, Faino L, Shi-Kunne X et al (2015) The genome of the saprophytic fungus Verticillium tricorpus reveals a complex effector repertoire resembling that of its pathogenic relatives. Mol Plant-Microbe Interact 28:362–373. https://doi.org/10.1094/MPMI-06-14-0173-R
Baroncelli R, Amby DB, Zapparata A et al (2016) Gene family expansions and contractions are associated with host range in plant pathogens of the genus Colletotrichum. BMC Genom 17:555
Arenas YC, Kalkman ERIC, Schouten A et al (2010) Functional analysis and mode of action of phytotoxic Nep1-like proteins of Botrytis cinerea. Physiol Mol Plant Pathol 74:376–386. https://doi.org/10.1016/j.pmpp.2010.06.003
Bashi ZD, Hegedus DD, Buchwaldt L et al (2010) Expression and regulation of Sclerotinia sclerotiorum necrosis and ethylene-inducing peptides (NEPs). Mol Plant Pathol 11:43–53. https://doi.org/10.1111/j.1364-3703.2009.00571.x
Motteram J, Küfner I, Deller S et al (2009) Molecular characterization and functional analysis of MgNLP, the sole NPP1 domain-containing protein, from the fungal wheat leaf pathogen Mycosphaerella graminicola. Mol Plant-Microbe Interact 22:790–799. https://doi.org/10.1094/MPMI-22-7-0790
Funding
This work was supported by The Minas Gerais State Foundation of Research Aid – FAPEMIG (Grant No. APQ-00150-17) and by The National Council of Scientific and Technological Development – CNPq (Fellowship Number PQ 302336/2019-2) to LOO. TCSD received student fellowships from the CAPES Foundation (PROEX – 0487 No. 1684083) and CNPq (GM/GD 142400/2018-1). HVSR was supported by a PD fellowship from the São Paulo Research Foundation – FAPESP (2018/04555-0).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception. TCSD performed data assembly and analyses. TCSD and LOO wrote the manuscript. HVSR commented and edited the manuscript. LOO and HVSR supervised the research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dal’Sasso, T.C.S., Rody, H.V.S. & Oliveira, L.O. Genome-Wide Analysis and Evolutionary History of the Necrosis- and Ethylene-Inducing Peptide 1-Like Protein (NLP) Superfamily Across the Dothideomycetes Class of Fungi. Curr Microbiol 80, 44 (2023). https://doi.org/10.1007/s00284-022-03125-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03125-8