Abstract
Many elements of a modern lifestyle influence the gut microbiota but few studies have explored the effect of physical health level. This study was aimed to explore the relationship between diet, physical health and gut microbiota in Chinese college students. A total of 69 college students were recruited, including 27 college athletes (AS group) and 42 healthy controls (HC group). Fecal samples were collected for 16S rRNA sequencing. According to National Standards for Students’ Physical Health (2014 revision), physical fitness measurements, dietary intake and health-related data were collected via questionnaires. ①According to the physical fitness scores, the physical fitness level of AS group was significantly higher than that of HC group (P < 0.05), there were no significant differences between the two groups in the frequency of intake of food. The frequency and duration of physical activity in the AS group were higher than those in the HC group (P < 0.05); ②The proportion and relative abundances of microorganism composition is varying at two groups: on the phylum level, AS group had mainly increased Firmicutes, Actinobacteria and reduced Bacteroidetes, Proteobacteria; on the genus level, AS group had mainly increased Faecalibacterium, Bifidobacterium and reduced Bacteroides; ③The associations with the 10 most abundant bacterial genera and physical fitness, dietary factors were investigated. Changes in the gut microbiota abundance can be sometimes reflective of a physical health status. Loss of the balance of gut microbial populations will lead to flora disorders and diseases. Therefore, further studies are needed to reveal the mechanisms behind the gut microbiota in its potential role.





Similar content being viewed by others
Data Availability
(The 16S rRNA amplicon sequences have been submitted to the Sequence Read Archive (SRA) database under the accession number PRJNA785678).
Code Availability
Not applicable.
References
Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG (2014) Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158(4):705–721
Tian R, Wang ZH, Dong YH, Yang ZP, Ma J (2017) A cross-section study on physical endurance level in primary and middle school students in China, 2014. Zhonghua Liu Xing Bing Xue Za Zhi 38(5):592–596
Wei CN, Harada K, Ueda K, Fukumoto K, Minamoto K, Ueda A (2012) Assessment of health-promoting lifestyle profile in Japanese university students. Environ Health Prev Med 17:222–227
Li XY, Jiang Y, Hu N, Li YC, Zhang M, Huang ZJ, Zhao WH (2012) Prevalence and characteristic of overweight and obesity among adults in China, 2010. Zhonghua yu fang yi xue za zhi [Chinese J prev med] 46(8):683–686
Logue JB, Stedmon CA, Kellerman AM, Nielsen NJ, Andersson AF (2016) Experimental insights into the importance of aquatic bacterial community composition to the degradation of dissolved organic matter. ISME J 10(3):533–545
Dong Y, Lau PWC, Dong B, Zou Z, Yang Y, Wen B, Ma Y (2019) Trends in physical fitness, growth, and nutritional status of Chinese children and adolescents: a retrospective analysis of 1·5 million students from six successive national surveys between 1985 and 2014. Lancet Child adolesc health 3(12):871–880
Guddal MH, Stensland S, Småstuen MC, Johnsen MB, Zwart JA, Storheim K (2019) Physical activity and sport participation among adolescents: associations with mental health in different age groups results from the young-HUNT study: a cross-sectional survey. BMJ Open 9(9):e028555
Kyral AM, Shipherd AM, Hearon CM (2019) The effect of moderate intensity aerobic exercise on affect and exercise intention in active and inactive college students. Int J Exerc Sci 12(5):1070–1079
Flint HJ, Scott KP, Louis P, Duncan SH (2012) The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 9(10):577–589
Moszak M, Szulińska M, Bogdański P (2020) You are what you eat-the relationship between diet, microbiota, and metabolic disorders-a review. Nutrients 12(4):1096
Wilson AS, Koller KR, Ramaboli MC, Nesengani LT, Ocvirk S, Chen C et al (2020) Diet and the human gut microbiome: an international review. Dig Dis Sci 65(3):723–740
Sheflin AM, Melby CL, Carbonero F, Weir TL (2017) Linking dietary patterns with gut microbial composition and function. Gut Microbes 8(2):113–129
Codella R, Luzi L, Terruzzi I (2018) Exercise has the guts: how physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig Liver Dis 50(4):331–341
Castellanos N, Diez GG, Antúnez-Almagro C, Bailén M, Bressa C, González Soltero R, Pérez M, Larrosa M (2020) A critical mutualism—competition interplay underlies the loss of microbial diversity in sedentary lifestyle. Front Microbiol 10:3142
Tabone M, Bressa C, García-Merino JA, Moreno-Pérez D, Van EC, Castelli FA, Fenaille F, Larrosa M (2021) The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Sci Rep 11(1):3558
Morita E, Yokoyama H, Imai D, Takeda R, Ota A, Kawai E, Hisada T, Emoto M, Suzuki Y, Okazaki K (2019) Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients 1(4):868
Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A et al (2014) Exercise and associated dietary extremes impact on gut microbial diversity. Gut 63(12):1913–1920
Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD et al (2019) Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med 25(7):1104–1109
Ticinesi A, Nouvenne A, Cerundolo N, Catania P, Prati B, Tana C, Meschi T (2019) Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients 11(7):1633
Description of national student physical health standards. 2014 Student physical health network.07.28. http://www.csh.moe.edu.cn/wtzx/bz/20141226/2c909e854a84301a014a8440085e000d.html
Steenbarger BN (1996) Research: the cornerstone of college health’s future. J Am Coll Health 44(5):193–218
Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD (2018) Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 50(4):747–757
Asano RY, Sales MM, Browne RA, Moraes JF, Coelho Júnior HJ, Moraes MR, Simões HG (2014) Acute effects of physical exercise in type 2 diabetes: a review. World J Diabetes 5(5):659–665
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60
Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ (2015) Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol 30(7):529–542
Ladabaum U, Mannalithara A, Myer PA, Singh G (2014) Obesity, abdominal obesity, physical activity, and caloric intake in US adults: 1988 to 2010. Am J Med 127(8):717-727.e712
Colley RC, Butler G, Garriguet D, Prince SA, Roberts KC (2018) Comparison of self-reported and accelerometer-measured physical activity in Canadian adults. Health Rep 29(12):3–15
Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, Bauman A (2016) Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet (London, England) 388(10051):1302–1310
Sasayama K, Adachi M (2019) Tracking of objective physical activity and physical fitness in Japanese children. BMC Res Notes 12(1):252
Ortiz-Alvarez L, Xu H, Martinez-Tellez B (2020) Influence of exercise on the human gut microbiota of healthy adults: a systematic review. Clin Transl Gastroenterol 11(2):e00126
Barton W, Penney NC (2018) The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 67(4):625–633
Durk RP, Castillo E, Márquez-Magaña L, Grosicki GJ, Bolter ND, Lee CM, Bagley JR (2019) Gut microbiota composition is related to cardiorespiratory fitness in healthy young adults. Int J Sport Nutr Exerc Metab 29(3):249–253
Qiu Q, Zhu Y, Qiu X, Gao C, Wang J, Wang H, He Y, Rahman MAU, Cao B, Su H (2019) Dynamic variations in fecal bacterial community and fermentation profile of holstein steers in response to three stepwise density diets. Animals (Basel) 9(8):560
Bárcena C, Valdés-Mas R, Mayoral P, Garabaya C, Durand S, Rodríguez F, Fernández-García MT (2019) Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat med 25(8):1234–1242
Chen X, Cui J, Zhang Y, Peng W (2020) The association between BMI and health-related physical fitness among Chinese college students: a cross-sectional study. BMC Public Health 20(1):444
Cho M, Kim JY (2017) Changes in physical fitness and body composition according to the physical activities of Korean adolescents. J Exerc Rehabil 13(5):568–572
Van Hul M, Le Roy T (2020) From correlation to causality: the case of Subdoligranulum. Gut Microbes 12(1):1–13
Zhao H, Xu H (2021) Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J Gastroenterol Hepatol 36(2):320–328
Gong D, Gong X, Wang L, Yu X, Dong Q (2016) Involvement of reduced microbial diversity in inflammatory bowel disease. Gastroenterol Res Pract 2016:6951091
Rajendiran E, Ramadass B, Ramprasath V (2021) Understanding connections and roles of gut microbiome in cardiovascular diseases. Can J Microbiol 67(2):101–111
Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, MICRO-Obes Consortium, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K (2016) Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65(3):426–436
Zhang Z, Mocanu V, Cai C, Dang J, Slater L, Deehan EC, Walter J, Madsen KL (2019) Impact of fecal microbiota transplantation on obesity and metabolic syndrome-a systematic review. Nutrients 11(10):2291
Henning SM, Yang J, Shao P, Lee RP, Huang J, Ly A, Hsu M (2017) Health benefit of vegetable/fruit juice-based diet: role of microbiome. Sci Rep 7(1):2167
Rastelli M, Cani PD, Knauf C (2019) The gut microbiome influences host endocrine functions. Endocr Rev 40(5):1271–1284
Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD, Woods JA (2018) Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 50(4):747–757
Schroeder BO, Birchenough GMH, Ståhlman M, Arike L, Johansson MEV, Hansson GC, Bäckhed F (2018) Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 23(1):27-40.e7
Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58(8):1091–1103
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110(22):9066–9071
Amabebe E, Robert FO, Agbalalah T, Orubu ESF (2020) Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br J Nutr 123(10):1127–1137
Van Hul M, Le Roy T, Prifti E, Dao MC, Paquot A, Zucker JD, Delzenne NM, Muccioli G, Clément K, Cani PD (2020) From correlation to causality: the case of Subdoligranulum. Gut Microbes 12(1):1–13
Zhao H, Xu H, Chen S, He J, Zhou Y, Nie Y (2021) Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J Gastroenterol Hepatol 36(2):320–328
Henning SM, Yang J, Shao P, Lee RP, Huang J, Ly A, Hsu M, Lu QY, Thames G, Heber D, Li Z (2017) Health benefit of vegetable/fruit juice-based diet: role of microbiome. Sci Rep 7(1):2167
Acknowledgements
We thank the staff of the College of physical education belonged to, Wuhan University of Science and Technology, for their kind cooperation. At the same time, thanks to others who contributed to the experiment but not been listed as authors.
Funding
The research was supported by Key research project of philosophy and social sciences of Hubei Provincial Department of Education in 2020 (Project No. 20D026); the National Undergraduate Innovation and Entrepreneurship Training Project (Project Nos. 202010488014 and JCX201976); 2020 General Planning Fund Project for Humanities and Social Sciences of the Ministry of Education, China (Project No. 20YJA880053); WUST National Defence Pre-research Foundation, China (Project No. GF202003), Hubei Province Key Laboratory Open Fund (Project No.OHIC2018Y05); Hubei Province College students Innovation and Entrepreneurship Training program (Project No. 201810488062) and Hubei Province Key Laboratory of Occupational Hazard Identification and Control, Wuhan University of Science and Technology (Project No. OHIC2020G05).
Author information
Authors and Affiliations
Contributions
Conceptualization: JQG and WXL. Funding: XFH, JQG, QW and CH. Investigation: SYC and JDW. Resources: JHT. Data curation: XCX and XQL. Writing–original draft: XFH. Writing–review & editing: QW, RS and QMW. Supervision: WZ and HC. All authors reviewed and accepted the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Medical Ethics Committee of Wuhan University of Science and Technology School of Medicine (Approval No. 202189). All participants provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
284_2022_3055_MOESM1_ESM.tif
Supplementary file1 (TIF 29151 kb) Fig. S1 The physical fitness scores of AS group (n = 27) were significantly higher than those of HC group (n = 42).
284_2022_3055_MOESM2_ESM.tif
Supplementary file2 (TIF 18800 kb) Fig. S2 Composition of gut microbiota in AS group and HC group at the phylum level (a), and genus level (b).
284_2022_3055_MOESM3_ESM.tif
Supplementary file3 (TIF 20314 kb) Fig. S3 Correlation analysis between the AS and HC group. (a) Correlation network graph Nodes in the network represented the predominant genera, and the connection between nodes indicates the correlation between the two genera. The thickness of the line indicates the strength of the correlation, and the thicker the line, the stronger the correlation. Solid lines indicate a positive correlation and dashed lines indicate a negative correlation. (b) Correlation heat map. The gradient from blue to red reflects the change in correlation from positive to negative.
284_2022_3055_MOESM4_ESM.tif
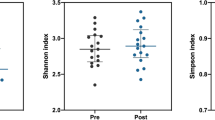
Supplementary file4 (TIF 6840 kb) Fig. S4 Structure of the gut microbiomes in three subgroups. (a) Alpha diversity indices, such as Chao1 richness(P = 0.55), Shannon diversity index(P = 0.54), and Simpson index(P = 0.67) of each group. (b) Beta diversity analysis among three subgroups of AS group. principal component analysis(P = 0.08), unweighted principal coordinate analysis(P = 0.632) and weighted principal coordinate analysis(P = 0.381).
284_2022_3055_MOESM5_ESM.tif
Supplementary file5 (TIF 18486 kb) Fig. S5 LEfSe analysis of intestinal microflora enrichment in three subgroups. Red represents excellent group (EX), blue represents good group (GD), and green represents pass group (PA). (a) The histogram shows the LDA score computed for genera differentially abundant between three subgroups of AS group and identified using LEfSe. (b) Cladogram showing the most differentially abundant taxa identified by LEfSe.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, X., Guo, J., Wang, J. et al. Study on the Relationship Between Diet, Physical Health and Gut Microflora of Chinese College Students. Curr Microbiol 79, 370 (2022). https://doi.org/10.1007/s00284-022-03055-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03055-5