Abstract
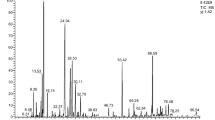
Owing to the resistance of nosocomial pathogens to antibiotics, the need for herbal medicines is felt. The aim of this study was to identify the chemical composition of bark essential oils of Campsis radicans and the effect of its free and encapsulated form on resistant nosocomial pathogens. This plant is a native of Northern Iran. The Bark essential oils of Campsis radicans was first extracted and its antimicrobial effects were investigated. Then, its phytochemical compounds were determined using Gas Chromatography−Mass Spectrometry (GC/MS). Guaiacol (2-methoxy phenol) was selected as the active ingredient among 32 compounds (2.40%). It was encapsulated and the encapsulation efficiency (EE), the particle size, polydispersity index (pdi), Fourier transform infrared (FTIR), release, and stability were determined. Then, the antimicrobial effect of both free and encapsulated forms was evaluated on cotrimoxazole-resistant Pseudomonas aeruginosa, cefixime-resistant Escherichia coli, and fluconazole-resistant Candida albicans. It was observed that both free and encapsulated forms of Guaiacol had an antimicrobial effect on the studied resistant strains, but the encapsulated form had a more antimicrobial effect due to more stability and a more targeted effect. MBC (MFC) ranged from 0.270 to 0.439 µg/ml in the free form and from 0.055 to 0.133 µg/ml in the encapsulated form, EE was 86%, particle size, and pdi were 138 nm and 0.26, respectively. This study showed that this plant can be a suitable alternative to chemical drugs due to its antimicrobial effects.





Similar content being viewed by others
Data Availability
All data are included in the manuscript.
Code Availability
Not applicable.
References
Alizadeh-Behbahani B, Tabatabaei-Yazdi F (2015) In vitro study of antibacterial activity of mangle negro extracts against selected pathogens from enterobacteriaceae and bacillaceae families. Zahedan J Res Med Sci In Press. https://doi.org/10.17795/zjrms-5202
Noshad M, Hojjati M, Alizadeh Behbahani B (2018) Black Zira essential oil: chemical compositions and antimicrobial activity against the growth of some pathogenic strain causing infection. Microb Pathog 116:153–157. https://doi.org/10.1016/j.micpath.2018.01.026
Tabatabaei-Yazdi F, Alizadeh-Behbahani B, Zanganeh H (2015) The Comparison among antibacterial activity of mespilus germanica extracts and number of common therapeutic antibiotics “in vitro.” Zahedan J Res Med Sci In Press. https://doi.org/10.17795/zjrms-5190
Dorman HJD, Deans SG (2000) Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 88:308–316. https://doi.org/10.1046/j.1365-2672.2000.00969.x
Larypoor M, Frsad S (2011) Evaluation of nosocomial infections in one of hospitals of Qom, 2008. Iran J Med Microbiol 5:7–17
Moein M, Zomorodian K, Almasi M et al (2017) Preparation and analysis of Rosa damascena essential oil composition and antimicrobial activity assessment of related fractions. Iran J Sci Technol Trans A 41:87–94. https://doi.org/10.1007/s40995-017-0220-2
Rossiter SE, Fletcher MH, Wuest WM (2017) Natural products as platforms to overcome antibiotic resistance. Chem Rev 117:12415–12474
Gherraf N, Zellagui A, Kabouche A et al (2017) Chemical constituents and antimicrobial activity of essential oils of Ammodaucus leucotricus. Arab J Chem 10:S2476–S2478. https://doi.org/10.1016/j.arabjc.2013.09.013
Siddique S, Parveen Z, Firdaus-e-Bareen MS (2020) Chemical composition, antibacterial and antioxidant activities of essential oils from leaves of three Melaleuca species of Pakistani flora. Arab J Chem 13:67–74. https://doi.org/10.1016/j.arabjc.2017.01.018
Fazly Bazzaz BS, Khameneh B, Namazi N et al (2018) Solid lipid nanoparticles carrying Eugenia caryophyllata essential oil: the novel nanoparticulate systems with broad-spectrum antimicrobial activity. Lett Appl Microbiol 66:506–513
Khalili ST, Mohsenifar A, Beyki M et al (2015) Encapsulation of Thyme essential oils in chitosan-benzoic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. LWT Food Sci Technol 60:502–508. https://doi.org/10.1016/j.lwt.2014.07.054
Radünz M, Luiza M, Camargo TM et al (2018) Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum L.) essential oil. Food Chem. https://doi.org/10.1016/j.foodchem.2018.09.173
Wen J, Jansen RK (1995) Morphological and molecular comparisons of Campsis grandiflora and C. radicans (Bignoniaceae), an eastern Asian and eastern North American vicariad species pair. Plant Syst Evol 196:173–183. https://doi.org/10.1007/BF00982958
Islam M, Jannat T, Kuddus MR et al (2019) In vitro and in vivo evaluation of pharmacological potentials of Campsis radicans L. Clin Phytosci. https://doi.org/10.1186/s40816-019-0144-9
Panda SP, Panigrahy UP, Panda S, Jena BR (2019) Stem extract of Tabebuia chrysantha induces apoptosis by targeting sEGFR in Ehrlich Ascites Carcinoma. J Ethnopharmacol 235:219–226
Sobiyana P, Anburaj G, Manikandan R (2019) Comparative analysis of the in vitro antioxidant antivity of Tabebuia rosea. and Tabebuia argentea. J Pharmacogn Phytochem 8:2673–2677
Mohammadi S, Sharifi-rad J, Asgharian P, Martorell M (2020) Medicinal plants used in the treatment of malaria: a key emphasis to Artemisia. Cinchona, Cryptolepis, and Tabebuia Genera. https://doi.org/10.1002/ptr.6628
Kiarsi Z, Hojjati M, Behbahani BA, Noshad M (2020) In vitro antimicrobial effects of Myristica fragrans essential oil on foodborne pathogens and its influence on beef quality during refrigerated storage. J Food Saf. https://doi.org/10.1111/jfs.12782
Beladi M, Akhavansepahy A, Mehrabian S, Esmaili A, Sharifnia F (2020) Evaluation of antimicrobial role of nanocapsules containing Cornus mass extract synthesized by emulsion method on antibiotic resistant bacteria. J Med Plants 19:84–107. https://doi.org/10.29252/jmp.19.74.84
Liu C, Liang B, Shi G et al (2015) Preparation and characteristics of nanocapsules containing essential oil for textile application. Flavour Fragr J 30:295–301. https://doi.org/10.1002/ffj.3245
Sorourian R, Eghbal A, Mehrnoosh K et al (2022) Structural characterization and cytotoxic, ACE—inhibitory and antioxidant activities of polysaccharide from Bitter vetch (Vicia ervilia ) seeds. J Food Measure Charact. https://doi.org/10.1007/s11694-022-01512-0
Sebaaly C, Charcosset C, Stainmesse S et al (2016) Clove essential oil-in-cyclodextrin-in-liposomes in the aqueous andlyophilized states: from laboratory to large scale using a membranecontactor. Carbohyd Polym 138:75–85. https://doi.org/10.1016/j.carbpol.2015.11.053
Shetta A, Kegere J, Mamdouh W (2018) Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int J of Biol Macromol 126:731–742. https://doi.org/10.1016/j.ijbiomac.2018.12.161
Sayout A, Ouarhach A, Dilagui I et al (2019) Antibacterial activity and chemical composition of essential oil from Lavandula tenuisecta Coss.ex Ball. an endemic species from Morocco. Eur J Integr Med 33:101017. https://doi.org/10.1016/j.eujim.2019.101017
Bhattacharjee I, Ghosh A, Chandra G (2005) Antimicrobial activity of the essential oil of Cestrum diurnum (L.) (Solanales: Solanaceae). Afr J Biotech 4:371–374. https://doi.org/10.4314/ajb.v4i4.15109
Anthony SJ, Zuchowski W, Setzer WN (2009) Composition of the floral essential oil of Brugmansia suaveolens. Rec Nat Prod 3(2):76
Snoussi M, Noumi E, Punchappady-Devasya R et al (2018) Antioxidant properties and anti-quorum sensing potential of Carum copticum essential oil and phenolics against Chromobacterium violaceum. J Food Sci Technol 55:2824–2832. https://doi.org/10.1007/s13197-018-3219-6
Esmaeili A, Saremnia B (2012) Preparation of extract-loaded nanocapsules from Onopordon leptolepis DC. Ind Crops Prod 37:259–263. https://doi.org/10.1016/j.indcrop.2011.12.010
Li Y, Kong D, Lin X et al (2016) Quality evaluation for essential oil of cinnamomum verum leaves at different growth stages based on GC–MS. FTIR and Microsc. https://doi.org/10.1007/s12161-015-0187-6
Souguir H, Salaün F, Douillet P et al (2013) Nanoencapsulation of curcumin in polyurethane and polyurea shells by an emulsion diffusion method. Chem Eng J 221:133–145. https://doi.org/10.1016/j.cej.2013.01.069
Ciko L, Andoni A, Ylli F et al (2016) Extraction of essential oil from albanian Salvia officinalis L. and its characterization by FTIR spectroscopy. Asian J Chem 28:1401–1402. https://doi.org/10.14233/ajchem.2016.19658
Yakhchi V, Jahanizadeh S, Yazdian F et al (2020) Synthesis and evaluation of lipid-based nanoparticle containing ginger extract against aspergillus species. J Shahid Sadoughi Univ Med Sci. https://doi.org/10.18502/ssu.v28i6.4155
Akbarzadeh I, Shayan M, Bourbour M et al (2021) Preparation, optimization and in-vitro evaluation of curcumin-loaded niosome@ calcium alginate nanocarrier as a new approach for breast cancer treatment. Biology 10:173
Vishwakarma GS, Gautam N, Babu JN, Jaitak V (2016) Polymeric encapsulates of essential oils and their constituents : a review of preparation techniques, characterization, and sustainable release mechanisms polymeric encapsulates of essential oils and their constituents. Polym Rev 56:668–701. https://doi.org/10.1080/15583724.2015.1123725
Jobdeedamrong A, Jenjob R, Crespy D, Accepted J (2018). Encapsulation and release of essential oils in functional silica nanocontainers. https://doi.org/10.1021/acs.langmuir.8b01652
Mahmoudzadeh M, Hosseini H, Shahraz F et al (2017) Essential oil composition and antioxidant capacity of carum copticum and its antibacterial effect on Staphylococcus aureus, Enterococcus faecalis and Escherichia coli O157:H7. J Food Process Preserv 41:1–11. https://doi.org/10.1111/jfpp.12938
Rakmai J, Cheirsilp B, Carlos J et al (2018) Antioxidant and antimicrobial properties of encapsulated guava leaf oil in hydroxypropyl-beta-cyclodextrin. Ind Crops Prod 111:219–225. https://doi.org/10.1016/j.indcrop.2017.10.027
Essien EE, Ogunwande IA, Setzer WN, Ekundayo O (2012) Chemical composition, antimicrobial, and cytotoxicity studies on S. erianthum and S. macranthum essential oils. Pharm Biol 50:474–480. https://doi.org/10.3109/13880209.2011.614623
Ahmadi Roudbaraki Z, Ranji N, Soltani Tehrani B (2017) Deregulation of mexb gene in ciprofloxacin resistant isolates of pseudomonas aeruginosa treated with silibinin-encapsulated in nanoparticles. J Babol Univ Med Sci 19:42–49
Ghomi Z, Tafvizi F, Naseh V, Akbarzadeh I (2020) Effect of Artemisia ciniformis extract on expression of NorA efflux pump gene in ciprofloxacin resistant Staphylococcus aureus by real time PCR. Iran J Med Microbiol 14:55–69
Rahimzadeh-Torabi L, Doudi M, Naghsh N, Golshani Z (2016) Comparing the antifungal effects of gold and silver nanoparticles isolated from patients with vulvovaginal candidiasis in-vitro. KAUMS J (FEYZ) 20:331–339
Ghaderi RS, Kazemi M, Soleimanpour S (2021) Nanoparticles are more successful competitor than antibiotics in treating bacterial infections: a review of the literature. Iran J Med Microbiol 15:18–45
Rashidian S, Mirzaie A et al (2018) Antibacterial and anticancer activities of biosynthesized silver nanoparticles using Artemisia khorassanica extract: Bax and Bcl2 apoptosis gene expression analysis. SSU J 26:1039–1049
Du SS, Yang K, Wang CF et al (2014) Chemical constituents and activities of the essential oil from myristica fragrans against cigarette beetle lasioderma serricorne. Chem Biodivers 11:1449–1456. https://doi.org/10.1002/cbdv.201400137
Van NH, Meile J-C, Lebrun M et al (2018) Litsea cubeba leaf essential oil from Vietnam: chemical diversity and its impacts on antibacterial activity. Lett Appl Microbiol 66:207–214
Esmaeili A, Rahnamoun S, Sharifnia F (2013) Effect of O/W process parameters on Crataegus azarolus L nanocapsule properties. J Nanobiotechnol 11:1–9. https://doi.org/10.1186/1477-3155-11-16
Kalhor N, Mirzaie A, Hamdi SMM (2021) Anti-biofilm effects of zinc oxide nanoparticles synthesized by leaf extract of typha latifolia on biofilm gene expression in multidrug-resistant klebsiella pneumoniae strains: a laboratory study. J Rafsanjan Univ Med Sci 20:3–22
Acknowledgements
The authors of this article express their gratitude for receiving technical assistance from the Islamic Azad University, North Tehran Branch.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
MR: Investigation (equal), Methodology (equal), Writing-original draft (equal); FS: Project administration (equal), Supervision (equal); ML: Supervision (equal), Writing-review & editing (equal); MM: Conceptualization (equal), Data curation (equal); SZ: Conceptualization (equal), Data curation (equal).
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramtin, M., Sharifniya, F., Larypoor, M. et al. Evaluation of the Active Ingredient of Campsis radicans Essential Oils and its Antimicrobial Evaluation Against Pathogenic Bacteria. Curr Microbiol 79, 338 (2022). https://doi.org/10.1007/s00284-022-03042-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03042-w