Abstract
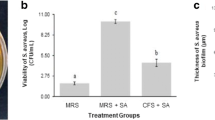
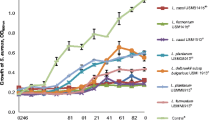
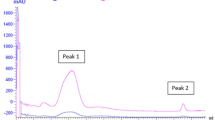
This study aims to evaluate the effects of bioactive metabolites produced by lactic acid bacteria against methicillin-resistant Staphylococcus aureus (MRSA) ATCC 43300. A total of six lactic acid bacteria (LAB) were selected to evaluate the antimicrobial activity against MRSA ATCC 43300, a skin pathogen that is highly resistant to most antibiotics. The K014 isolate from a fermented vegetable recorded the highest inhibition against MRSA ATCC 43300 at 91.93 ± 0.36%. 16S rRNA sequencing revealed the K014 isolate is closely related to L. plantarum and the sequence was subsequently deposited in the GenBank database with an accession number of MW180960, named as Lactiplantibacillus plantarum K014. The cell-free supernatant (CFS) of L. plantarum K014 had tolerance to high temperature as well as acidic pH. The bioactive metabolites, such as hydrogen peroxide, lactic acid and hyaluronic acid, were produced by L. plantarum K014. Result from ABTS assay showed higher antioxidant activity (46.28%) as compared to that obtained by DPPH assay (2.97%). The CFS had showed anti-inflammatory activity for lipoxygenase (LOX) assay at 43.66%. The bioactive metabolites of L. plantarum K014 showed very promising potential to be used topical skin pathogens.


Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Swetha TK, Pandian SK (2019) Role of bacteria in dermatological infections. In: Pocket guide to bacterial infections, p 279
Otto M (2010) Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev Dermatol 5(2):183–195
Foster TJ, Geoghegan JA, Ganesh VK, Höök M (2014) Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12(1):49–62
Liu J, Chen D, Peters BM, Li L, Li B, Xu Z, Shirliff ME (2016) Staphylococcal chromosomal cassettes mec (SCCmec): a mobile genetic element in methicillin-resistant Staphylococcus aureus. Microb Pathog 101:56–67
Rodvold KA, McConeghy KW (2014) Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis 58(suppl_1):S20–S27
Ong JS, Taylor TD, Yong CC, Khoo BY, Sasidharan S, Choi SB et al (2020) Lactobacillus plantarum USM8613 aids in wound healing and suppresses Staphylococcus aureus infection at wound sites. Probiotics Antimicrob Proteins 12(1):125–137
Ishikawa T, Imai S, Nakano T, Terai T, Okumura T, Hanada N, Kawahara H (2020) Antibacterial activity of the probiotic candidate Lactobacillus gasseri against methicillin-resistant Staphylococcus aureus. Asian Pac J Dent 20(1):1–8
Sürmeli M, Maçin S, Akyön Y, Kayikçioğlu AU (2019) The protective effect of Lactobacillus plantarum against meticillin-resistant Staphylococcus aureus infections: an experimental animal model. J Wound Care 28(Sup3b):s29–s34
Devi SM, Aishwarya S, Halami PM (2016) Discrimination and divergence among Lactobacillus plantarum-group (LPG) isolates with reference to their probiotic functionalities from vegetable origin. Syst Appl Microbiol 39(8):562–570
Vataščinová T, Pipová M, Fraqueza M, Maľa P, Dudriková E, Drážovská M, Lauková A (2020) Antimicrobial potential of Lactobacillus plantarum strains isolated from Slovak raw sheep milk cheeses. J Dairy Sci 103(8):6900–6903
Ng ZJ, Zarin MA, Lee CK, Phapugrangkul P, Tan JS (2020) Isolation and characterization of Enterococcus faecium DSM 20477 with ability to secrete antimicrobial substance for the inhibition of oral pathogen Streptococcus mutans UKMCC 1019. Arch Oral Biol 110:104617
Azrad M, Tkhawkho L, Isakovich N, Nitzan O, Peretz A (2018) Antimicrobial susceptibility of Campylobacter jejuni and Campylobacter coli: comparison between Etest and a broth dilution method. Ann Clin Microbiol Antimicrob 17(1):1–5
Jang HJ, Song MW, Lee N-K, Paik H-D (2018) Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum Ln1 isolated from kimchi. J Food Sci Technol 55(8):3174–3180
Son S-H, Jeon H-L, Jeon EB, Lee N-K, Park Y-S, Kang D-K, Paik H-D (2017) Potential probiotic Lactobacillus plantarum Ln4 from kimchi: Evaluation of β-galactosidase and antioxidant activities. LWT-Food Sci Technol 85:181–186
Douros A, Hadjipavlou-Litina D, Nikolaou K, Skaltsa H (2018) The occurrence of flavonoids and related compounds in Cedrus brevifolia A. Henry ex Elwes & A. Henry needles. Inhibitory potencies on lipoxygenase, linoleic acid lipid peroxidation and antioxidant activity. Plants 7(1):1
Liyanaarachchi GD, Samarasekera JKRR, Mahanama KRR, Hemalal KDP (2018) Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind Crops Prod 111:597–605
Borshchevskaya L, Gordeeva T, Kalinina A, Sineokii S (2016) Spectrophotometric determination of lactic acid. J Anal Chem 71(8):755–758
Lew L-C, Gan C-Y, Liong M-T (2013) Dermal bioactives from lactobacilli and bifidobacteria. Ann Microbiol 63(3):1047–1055
Murtey MD, Ramasamy P (2016) Sample preparations for scanning electron microscopy–life sciences. Mod Electron Microsc Phys Life Sci 161–185
Yi H, Han X, Yang Y, Liu W, Liu H, Zhang Y et al (2013) Effect of exogenous factors on bacteriocin production from Lactobacillus paracasei J23 by using a resting cell system. Int J Mol Sci 14(12):24355–24365
Sidooski T, Brandelli A, Bertoli SL, Souza, Carvalho LF CK (2019) Physical and nutritional conditions for optimized production of bacteriocins by lactic acid bacteria–a review. Crit Rev Food Sci Nutr 59(17):2839–2849
Suganthi V, Mohanasrinivasan V (2015) Optimization studies for enhanced bacteriocin production by Pediococcus pentosaceus KC692718 using response surface methodology. J Food Sci Technol 52(6):3773–3783
Muhammad Z, Ramzan R, Abdelazez A, Amjad A, Afzaal M, Zhang S, Pan S (2019) Assessment of the antimicrobial potentiality and functionality of Lactobacillus plantarum strains isolated from the conventional inner Mongolian fermented cheese against foodborne pathogens. Pathogens 8(2):71
Arrioja-Bretón D, Mani-López E, Palou E, López-Malo A (2020) Antimicrobial activity and storage stability of cell-free supernatants from lactic acid bacteria and their applications with fresh beef. Food Control 115:107286
Lin T-H, Pan T-M (2019) Characterization of an antimicrobial substance produced by Lactobacillus plantarum NTU 102. J Microbiol Immunol Infect 52(3):409–417
Hassan MU, Nayab H, Rehman TU, Williamson MP, Haq KU, Shafi N, Shafique F (2020) Characterisation of bacteriocins produced by Lactobacillus spp. isolated from the traditional Pakistani yoghurt and their antimicrobial activity against common foodborne pathogens. BioMed Res Int
Vieco-Saiz N, Belguesmia Y, Raspoet R, Auclair E, Gancel F, Kempf I, Drider D (2019) Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front Microbiol 10:57
Raftari M, Jalilian FA, Abdulamir A, Son R, Sekawi Z, Fatimah A (2009) Effect of organic acids on Escherichia coli O157: H7 and Staphylococcus aureus contaminated meat. Open Microbiol J 3:121
Md Sidek NL, Halim M, Tan JS, Abbasiliasi S, Mustafa S, Ariff AB (2018) Stability of bacteriocin-like inhibitory substance (BLIS) produced by Pediococcus acidilactici kp10 at different extreme conditions. BioMed Res Int
Shrivas P, Zodape S, Wankhade A, Pratap U (2020) Facile synthesis of benzazoles through biocatalytic cyclization and dehydrogenation employing catalase in water. Enzyme Microb Technol 138:109562
Atassi F, Servin AL (2010) Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC933 and vaginal strain Lactobacillus gasseri KS120. 1 against enteric, uropathogenic and vaginosis-associated pathogens. FEMS Microbiol Lett 304(1):29–38
Santos-Sánchez NF, Salas-Coronado R, Villanueva-Cañongo C, Hernández-Carlos B (2019) Antioxidant compounds and their antioxidant mechanism. IntechOpen, London
Son S-H, Yang S-J, Jeon H-L, Yu H-S, Lee N-K, Park Y-S, Paik H-D (2018) Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from Korean traditional fermented food, Jangajji. Microb Pathog 125:486–492
Yang S-J, Lee J-E, Lim S-M, Kim Y-J, Lee N-K, Paik H-D (2019) Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food science and biotechnology 28(2):491–499
Eshwarappa RSB, Ramachandra YL, Subaramaihha SR, Subbaiah SGP, Austin RS, Dhananjaya BL (2016) Anti-Lipoxygenase activity of leaf gall extracts of Terminalia chebula (Gaertn.) Retz. (Combretaceae). Pharmacogn Res 8(1):78
Makris G, Wright JD, Ingham E, Holland KT (2004) The hyaluronate lyase of Staphylococcus aureus–a virulence factor? Microbiology 150(6):2005–2013
Jiratchayamaethasakul C, Ding Y, Hwang O, Im S-T, Jang Y, Myung S-W et al (2020) In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish Aquat Sci 23(1):1–9
Buyankhishig B, Murata T, Suganuma K, Batkhuu J, Sasaki K (2020) Hyaluronidase inhibitory saponins and a trypanocidal isoflavonoid from the aerial parts of Oxytropis lanata. Fitoterapia 145:104608
Lei W, Liu C, Pan L, Peng C, Wang J, Zhou H (2021) Screening of probiotic Lactobacilli with potential anti-allergic activity based on hyaluronidase inhibition and degranulation of RBL-2H3 cells in vitro. LWT 140:110707
Chahuki FF, Aminzadeh S, Jafarian V, Tabandeh F, Khodabandeh M (2019) Hyaluronic acid production enhancement via genetically modification and culture medium optimization in Lactobacillus acidophilus. Int J Biol Macromol 121:870–881
Kranjec C, Ovchinnikov KV, Grønseth T, Ebineshan K, Srikantam A, Diep DB (2020) A bacteriocin-based antimicrobial formulation to effectively disrupt the cell viability of methicillin-resistant Staphylococcus aureus (MRSA) biofilms. NPJ Biofilms Microbiomes 6(1):1–13
Acknowledgements
The authors acknowledge the financial support received by Ministry of Higher Education Malaysia for Fundamental Research Grant Scheme with Project Code: FRGS/1/2022/STG01/USM/02/21.
Funding
This study was supported by Fundamental Research Grant Scheme (FRGS) by Ministry of Higher Education, Malaysia (Project Code: FRGS/1/2018/SKK11/USM/02/1).
Author information
Authors and Affiliations
Contributions
LKM conducted the experiments and wrote the manuscript. HWT and ZJN conducted the experiments and analyse the data. TP, KMH and AAKAS provided the facility to conduct the MRSA experiment. SA and JST provided experiment idea, revised the manuscript and supervised the research.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Accession Number
Lactiplantibacillus plantarum strain K014 16S ribosomal RNA gene, partial sequence MW180960.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mlambo, L.K., Abbasiliasi, S., Tang, H.W. et al. Bioactive Metabolites of Lactiplantibacillus plantarum K014 Against Methicillin-Resistant Staphylococcus aureus ATCC43300 and In Vitro Evaluation of Its Antibacterial, Antioxidant and Anti-inflammatory Activities. Curr Microbiol 79, 359 (2022). https://doi.org/10.1007/s00284-022-03038-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03038-6