Abstract
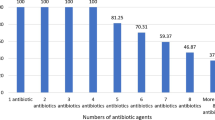
This study aimed to investigate the prevalence of Methicillin- and Vancomycin-Resistant Staphylococcus aureus (MRSA, VRSA) and Vancomycin-Resistant Enterococcus (VRE) of hospital food samples in Mashhad, Iran. A total of 357 hospital food samples were collected from 13 hospitals. Enterococcus spp. and Staphylococcus aureus were identified using conventional cultural techniques following genotypic confirmation by PCR. The antibiotic resistance patterns of MRSA, VRSA, and VRE strains were analyzed using the disk diffusion methods. The prevalence of S. aureus and MRSA were 24.37% (87/357) and 22.98% (20.87), respectively. In addition, the vanB gene involved in vancomycin resistance was detected in 1.14% of the S. aureus strains. Enterococci and VRE had a prevalence of 15.4% (55/357) and 21.81% (12/55), respectively. Meat, chicken barbecues, and salad were the most commonly contaminated samples with S. aureus, MRSA, Enterococci, and VRE. PCR detected two vancomycin resistance genes, including vanA (1.81%, 1.55) and vanC2 (20%, 11.55) genes. MRSA strains revealed the highest resistance against penicillin, erythromycin, clindamycin, azithromycin, tetracycline, and gentamicin. The VRSA isolates were resistant to penicillin, ampicillin, oxacillin, cefoxitin, clindamycin, erythromycin, gentamicin, and trimethoprim–sulfamethoxazole. Furthermore, VRE isolates exhibited the highest resistance against quinupristin-dalfopristin, erythromycin, and tetracycline. The results of this study indicated that hospital foods might act as a reservoir of Enterococci spp. and S. aureus strains, which can transfer antibiotic resistance. Moreover, multidrug resistance (MDR) in some MRSA, VRSA, and VRE isolates represents a serious threat to susceptible persons in hospitals.
Similar content being viewed by others
Data Availability
The dataset used is available from the corresponding author (email: saramohamadi12@yahoo.com), upon judicious request.
Code Availability
Not applicable.
References
Lund BM, O’Brien SJ (2009) Microbiological safety of food in hospitals and other healthcare settings. J Hosp Infect 73(2):109–120. https://doi.org/10.1016/j.jhin.2009.05.017
Ranjbar R, Masoudimanesh M, Dehkordi FS, Jonaidi-Jafari N, Rahimi E (2017) Shiga (Vero)-toxin producing Escherichia coli isolated from the hospital foods; virulence factors, o-serogroups and antimicrobial resistance properties. Antimicrob Resist Infect Control 6(1):1–11. https://doi.org/10.1186/s13756-016-0163-y
Safarpoor Dehkordi F, Basti AA, Gandomi H, Misaghi A, Rahimi E (2018) Retracted: Pathogenic Staphylococcus aureus in hospital food samples; prevalence and antimicrobial resistance properties. J Food Saf 38(6):e12501. https://doi.org/10.1111/jfs.12501
Dehkordi FS, Gandomi H, Basti AA, Misaghi A, Rahimi E (2017) Phenotypic and genotypic characterization of antibiotic resistance of methicillin-resistant Staphylococcus aureus isolated from hospital food. Antimicrob Resist Infect Control 6(1):1–11. https://doi.org/10.1186/s13756-017-0257-1
Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC (2008) Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis 46(5):668–674. https://doi.org/10.1086/527392
Banwo K, Sanni A, Tan H, Tian Y (2012) Phenotypic and genotypic characterization of lactic acid bacteria isolated from some Nigerian traditional fermented foods. Food Biotechnol 26(2):124–142. https://doi.org/10.1080/08905436.2012.670831
El-Zamkan MA, Mubarak AG, Ali AO (2019) Prevalence and phylogenetic relationship among methicillin-and vancomycin-resistant Staphylococci isolated from hospital’s dairy food, food handlers, and patients. J Adv Vet Anim Res 6(4):463. https://doi.org/10.5455/javar.2019.f369
Tang Y-T, Cao R, Xiao N, Li Z-S, Wang R, Zou J-M, Pei J (2018) Molecular epidemiology and antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus isolates in Xiangyang, China. J Glob Antimicrob Resist 12:31–36. https://doi.org/10.1016/j.jgar.2017.08.016
Raafat SA, Elmagd EKA, Awad RA, Hassan EM, Alrasheedy ZE (2016) Prevalence of vancomycin resistant enterococci in different food samples, Egypt. J Med Microbiol 25(4):47–55
Mahajan S, Gupta V (2017) Other bacterial infections: vancomycin-resistant enterococcus (VRE), methicillin-resistant Staphylococcus aureus (MRSA). Emerging infectious uveitis. Springer, Cham, pp 87–91
Moussally M, Zahreddine N, Kazma J, Ahmadieh R, Kan SS, Kanafan ZA (2021) Prevalence of antibiotic-resistant organisms among hospitalized patients at a tertiary care center in Lebanon, 2010–2018. J Infect Public Health 14(1):12–16. https://doi.org/10.1016/j.jiph.2020.11.006
Benjelloun Touimi G, Bennani L, Berrada S, Moussa B, Bennani B (2020) Prevalence and antibiotic resistance profiles of Staphylococcus sp. isolated from food, food contact surfaces and food handlers in a Moroccan hospital kitchen. Lett Appl Microbiol 70(4):241–251. https://doi.org/10.1111/lam.13278
Caniça M, Manageiro V, Abriouel H, Moran-Gilad J, Franz CM (2019) Antibiotic resistance in foodborne bacteria. Trends Food Sci Technol 84:41–44. https://doi.org/10.1016/j.tifs.2018.08.001
Konecka-Matyjek E, Mackiw E, Krygier B, Tomczuk K, Stos K, Jarosz M (2012) National monitoring study on microbial contamination of food-contact surfaces in hospital kitchens in Poland. Ann Agric Environ Med 19(3):1
Ayçıçek H, Sarimehmetoǧlu B, Çakiroǧlu S (2004) Assessment of the microbiological quality of meals sampled at the meal serving units of a military hospital in Ankara, Turkey. Food Control 15(5):379–384. https://doi.org/10.1016/S0956-7135(03)00101-4
Gholammostafaei F, Alebouyeh M, Jabari F, Asadzadehaghdaei H, Zali M, Solaimannejad K (2014) Prevalence of antibiotic resistant bacteria isolated from foodstuff in kitchen of a hospital in Tehran. J Ilam Univ Med Sci 22(2):1–9
Ranjbar R, Shahreza MHS, Rahimi E, Jonaidi-Jafari N (2017) Methicillin-resistant Staphylococcus aureus isolates from Iranian restaurant food samples: panton-valentine leukocidin, SCCmec phenotypes and antimicrobial resistance. Trop J Pharm Res 16(8):1939–1949. https://doi.org/10.4314/tjpr.v16i8.26
Shahraz F, Dadkhah H, Khaksar R, Mahmoudzadeh M, Hosseini H, Kamran M, Bourke PJMs, (2012) Analysis of antibiotic resistance patterns and detection of mecA gene in Staphylococcus aureus isolated from packaged hamburger. Meat sci 90(3):759–763. https://doi.org/10.1016/j.meatsci.2011.11.009
Guran HS, Kahya S (2015) Species diversity and pheno-and genotypic antibiotic resistance patterns of staphylococci isolated from retail ground meats. J Food Sci 80(6):M1291–M1298. https://doi.org/10.1111/1750-3841.12893
Al-Amery K, Elhariri M, Elsayed A, El-Moghazy G, Elhelw R, El-Mahallawy H, El Hariri M, Hamza D (2019) Vancomycin-resistant Staphylococcus aureus isolated from camel meat and slaughterhouse workers in Egypt. Antimicrob Resist Infect Control 8(1):1–8. https://doi.org/10.1186/s13756-019-0585-4
Costa WLR, Ferreira JdS, Carvalho JS, Cerqueira ES, Oliveira LC, Almeida RCdC (2015) Methicillin-resistant Staphylococcus aureus in raw meats and prepared foods in public hospitals in Salvador, Bahia, Brazil. J Food Sci 80(1):M147–M150. https://doi.org/10.4236/fns.2015.614138
Kwon JH, Reske KA, Hink T, Seiler SM, Wallace MA, Bommarito KM, Burnham C-AD, Dubberke ER (2017) An evaluation of the prevalence of VRE and MRSA in hospital food. Infect Control Hosp Epidemiol 38(11):1373. https://doi.org/10.1017/ice.2017.207
Shahid AH, Nazir KNH, El Zowalaty ME, Kabir A, Sarker SA, Siddique MP, Ashour HMJOH (2021) Molecular detection of vancomycin and methicillin resistance in Staphylococcus aureus isolated from food processing environments. One Health 1(13):100276. https://doi.org/10.1016/j.onehlt.2021.100276
Rahimi F, Shafiei R (2019) Characteristics of enterotoxin-producing methicillin-resistant Staphylococcus aureus strains isolated from meat in Tehran, Iran. J Consum Prot Food Safety 14(4):389–398. https://doi.org/10.1007/s00003-019-01239-z
Jackson CR, Davis JA, Barrett JB (2013) Prevalence and characterization of methicillin-resistant Staphylococcus aureus isolates from retail meat and humans in Georgia. J Clin Microbiol 51(4):1199–1207. https://doi.org/10.1128/JCM.03166-12
Rong D, Wu Q, Xu M, Zhang J, Yu S (2017) Prevalence, virulence genes, antimicrobial susceptibility, and genetic diversity of Staphylococcus aureus from retail aquatic products in China. Front Microbiol 8:714. https://doi.org/10.3389/fmicb.2017.00714
Saadat S, Solhjoo K, Norooz-Nejad M-J, Kazemi A (2014) VanA and vanB positive vancomycin-resistant Staphylococcus aureus among clinical isolates in Shiraz, South of Iran. Oman Med J 29(5):335. https://doi.org/10.5001/omj.2014.90
Rahimipour F, Roudbari F, Azimian A, Youssefi M, Ghazvini K (2015) Prevalence of Staphylococcus aureus with reduced susceptibility against vancomycin in clinical samples isolate from Mashhad hospitals during 2014. J North Khorasan Univ Med Sci 7(2):309–318. https://doi.org/10.29252/jnkums.7.2.309
Martins PD, de Almeida TT, Basso AP, de Moura TM, Frazzon J, Tondo EC, Frazzon APG (2013) Coagulase-positive staphylococci isolated from chicken meat: pathogenic potential and vancomycin resistance. Foodborne Pathog Dis 10(9):771–776. https://doi.org/10.1089/fpd.2013.1492
Soltan Dallal MM, Shojaei Zinjanab M, Vahedi S, Mahmoudi H, Ghanbarzadeh S, Hedayati Rad F (2016) A survey of Escherichia coli, Enterococcus and total microbial count of packaged and non-packaged fresh vegetables in Tehran. J Payavard Salamat 10(3):220–229
Franz CM, Holzapfel WH, Stiles ME (1999) Enterococci at the crossroads of food safety. Inter J Food Microbiol 47(1–2):1–24. https://doi.org/10.1016/S0168-1605(99)00007-0
Faramarzi T, JonidiJafari A, Dehghani S, Mirzabeygi M, Naseh M, RahbarArasteh H (2012) A survey on bacterial contamination of food supply in the west of Tehran. J Fasa Uni Med Sci 2(1):11–18
Mazaheri Nezhad Fard R, Soltan Dallal MM, Abbaspour M, Rajabi Z (2019) Study of VanA, B, C, D, E genes in vancomycin resistant enterococci isolated from retailed dried vegetables in Tehran, Iran. Int J Enteric Pathog 7(1):9–14. https://doi.org/10.15171/ijep.2019.03
Fracalanzza SAP, Scheidegger EMD, Santos PFd, Leite PC, Teixeira LM (2007) Antimicrobial resistance profiles of enterococci isolated from poultry meat and pasteurized milk in Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 102(7):853–859. https://doi.org/10.1590/s0074-02762007005000120
Serio A, Chaves-López C, Paparella A, Suzzi G (2010) Evaluation of metabolic activities of enterococci isolated from Pecorino Abruzzese cheese. Inter Dairy J 20(7):459–464. https://doi.org/10.1016/j.idairyj.2010.02.005
Novais C, Coque T, Costa M, Sousa J, Baquero F, Peixe L (2005) High occurrence and persistence of antibiotic-resistant enterococci in poultry food samples in Portugal. J Antimicrob Chemother 56(6):1139–1143. https://doi.org/10.1093/jac/dki360
Jahansepas A, Sharifi Y, Aghazadeh M, Ahangarzadeh Rezaee M (2020) Comparative analysis of Enterococcus faecalis and Enterococcus faecium strains isolated from clinical samples and traditional cheese types in the Northwest of Iran: antimicrobial susceptibility and virulence traits. Arch Microbiol 202(4):765–772. https://doi.org/10.1007/s00203-019-01792-z
Kim M-C, Cha M-H, Ryu J-G, Woo G-J (2017) Characterization of vancomycin-resistant Enterococcus faecalis and Enterococcus faecium isolated from fresh produces and human fecal samples. Foodborne Pathog Dis 14(4):195–201. https://doi.org/10.1089/fpd.2016.2188
Gousia P, Economou V, Bozidis P, Papadopoulou C (2015) Vancomycin-resistance phenotypes, vancomycin-resistance genes, and resistance to antibiotics of enterococci isolated from food of animal origin. Foodborne Pathog Dis 12(3):214–220. https://doi.org/10.1089/fpd.2014.1832
Torre I, Pennino F, Diana MV, De Marco G, Trotta A, Borriello T, Troiano E (2012) Antimicrobial susceptibility and glycopeptide-resistance of enterococci in vegetables. Ital J Pub Health. https://doi.org/10.2427/5746
Gaglio R, Couto N, Marques C, Lopes MdFS, Moschetti G, Pomba C, Settanni L (2016) Evaluation of antimicrobial resistance and virulence of enterococci from equipment surfaces, raw materials, and traditional cheeses. Int J Food Microbiol 236:107–114. https://doi.org/10.1016/j.ijfoodmicro.2016.07.020
Jung WK, Lim JY, Kwon NH, Kim JM, Hong SK, Koo HC, Kim SH, Park YH (2007) Vancomycin-resistant enterococci from animal sources in Korea. Int J Food Microbiol 113(1):102–107. https://doi.org/10.1016/j.ijfoodmicro.2006.07.023
Ristori CA, Rowlands REG, Bergamini AMM, Lopes GISL, Paula AMRd, Oliveira MAd, Lima MdJdC, Tegani LS, Watanabe AH, Jakabi M (2012) Prevalence and antimicrobial susceptibility profile of Enterococcus spp. isolated from frozen chicken carcasses. Rev Inst Adolfo Lutz (Impresso) 71(2):237–243
Madanipour E, Mehrabi MR, Mirzaee M (2017) The antibiotic susceptibility pattern and prevalence of vanA, vanB, and vanC Genes among Enterococcus faecalis strains isolated from consumed meat. Infect Epidemiol Microbiol 3(4):117–121
Acknowledgements
The authors sincerely appreciate all those who participated in this study.
Funding
The financial support acquired from Faculty of Medicine, Mashhad University of Medical Sciences (Grant No: A-1581).
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design, AA and MH; data collection, ST; analysis and interpretation of results, AA, ST, AN, SM, and MN; writing the manuscripts, SM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Ethical Approval
Not applicable.
Consent for Participate and Publication
The authors declared their total consent for participation and publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afshari, A., Taheri, S., Hashemi, M. et al. Methicillin- and Vancomycin-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococci Isolated from Hospital Foods: Prevalence and Antimicrobial Resistance Patterns. Curr Microbiol 79, 326 (2022). https://doi.org/10.1007/s00284-022-03022-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03022-0