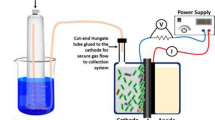
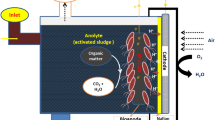
Abstract
Electroactive microorganisms play a significant role in microbial fuel cells (MFCs). These devices are environmentally friendly and can turn large quantities of organic material into renewable energy based on microbial diversity. Based on broad microbial diversity, it is necessary to obtain a comprehensive understanding of their resource distribution and to discover potential resources. In this study, sweet potato tissues were selected to isolate endophytic bacteria, and the electrochemical activity potential of those bacteria was evaluated by high-throughput screening with a WO3 nanoprobe. This study was screened and obtained a strain SHE10 with electrochemical performance from the rhizome of sweet potato by a WO3 nanoprobe, which was identified as Shinella zoogloeoides. After nearly 600 h of voltage monitoring and cyclic voltammetry analysis, the results showed that the average voltage of S. zoogloeoides SHE10 reached 122.5 mV in stationary period. The maximum power density is 78.3 ± 1.8 mW/m2, and the corresponding current density is 223.0 mA/m2. The good redox reaction also indicated that the strain had good electrical activity. Its electron transfer mode was diverse, but its power generation mechanism still needs to be further discussed. The study of S. zoogloeoides SHE10 provides scientific theoretical reference for expanding the resource pool of electroproducing bacteria and the types of electroproducing microorganisms.





Similar content being viewed by others
Data Availability
All test data in the article are transparent and materials are available.
Code Availability
Not applicable.
References
Allen RM, Bennetto HP (1993) Microbial fuel-cells - Electricity production from carbohydrates. Appl Biochem Biotechnol 39:27–40. https://doi.org/10.1007/BF02918975
Lovley DR (2008) The microbe electric: conversion of organic matter to electricity. Curr Opin Biotechnol 19:564–571. https://doi.org/10.1016/j.copbio.2008.10.005
Fan Y, Han SK, Liu H (2012) Improved performance of CEA microbial fuel cells with increased reactor size. Energy Environ Sci 5:8273–8280. https://doi.org/10.1039/C2EE21964F
Chen WW, Liu ZL, Li YX (2021) Improved electricity generation, coulombic efficiency and microbial community structure of microbial fuel cells using sodium citrate as an effective additive. J Power Sources 482:228947. https://doi.org/10.1016/j.jpowsour.2020.228947
Rossi R, Hur AY, Page MA (2022) Pilot scale microbial fuel cells using air cathodes for producing electricity while treating wastewater. Water Res 215:118208. https://doi.org/10.1016/j.watres.2022.118208
Zhang J, Chen RY, Du C (2021) Effects of continuous sulfamonomethoxine shock on the power generation performance and microbial community structure of MFCs under seasonal temperature variation. Biochem Eng J 167:107909. https://doi.org/10.1016/j.bej.2020.107909
Shen J, Li JF, Li FS (2021) Effect of lignite activated coke packing on power generation and phenol degradation in microbial fuel cell treating high strength phenolic wastewater. Chemical Engineering Journa 417:128091. https://doi.org/10.1016/j.cej.2020.128091
Chauhan S, Sharma V, Varjani S (2022) Mitigation of tannery effluent with simultaneous generation of bioenergy using dual chambered microbial fuel cell. Biores Technol 351:127084. https://doi.org/10.1016/j.biortech.2022.127084
Li M, Zhou MH, Tian XY (2018) Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol Adv 36:1316–1327. https://doi.org/10.1016/j.biotechadv.2018.04.010
Yuan SJ, He H, Sheng GP (2013) A photometric high-throughput method for identification of electrochemically active bacteria using a WO 3 nanocluster probe. Sci Rep 3:1–7. https://doi.org/10.1038/srep01315
Ling LJ, Li ZB, Yang CY (2020) Plant endophytic bacteria: a potential resource pool of electroactive microorganisms. Biorxiv 10:334912. https://doi.org/10.1101/2020.10.11.334912
Da Silva P, Nahas E (2002) Bacterial diversity in soil in response to different plants, phosphate fertilizers and liming. Brazilian J Microbiol 33:304–310. https://doi.org/10.1590/S1517-83822002000400005
Widdel F, Bak F (1992) Gram-negative mesophilic sulfate-reducing bacteria. In: The Prokaryotes. Springer New York, pp 3352–3378 https://doi.org/10.1007/978-1-4757-2191-1_21
Dudley K (1990) Short protocols in molecular biology. FEBS Lett 268:434–434. https://doi.org/10.1016/S0960-0760(97)80799-6
Ling LJ, Yang CY, Ma WX (2020) Isolation, identification and control of a resistant bacterium strain found in Ku shui rose pure dew. J Food Process Preserv 45:15061. https://doi.org/10.1111/jfpp.15061
Schloss PD, Westcott SL (2011) Assessing and improving methods used in operational taxonomic unit-based approaches for 16S rRNA gene sequence analysis. Appl Environ Microbiol 77:3219–3226. https://doi.org/10.1128/AEM.02810-10
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution (N Y) 39:783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Bond DR, Lovley DR (2003) Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69:1548–1555. https://doi.org/10.1128/AEM.69.3.1548-1555.2003
Logan B, Cheng S, Watson V, Estadt G (2007) Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ Sci Technol 41:3341–3346. https://doi.org/10.1021/es062644y
Xia X, Cao XX, Liang P (2010) Electricity generation from glucose by a Klebsiella sp. in microbial fuel cells. Appl Microbiol Biotechnol 87:383–390. https://doi.org/10.1007/s00253-010-2604-5
Bowon K (1999) Dynamic effects of learning capabilities and profit structures on the innovation competition. Optim Control Appl Methods 20:127–144. https://doi.org/10.1002/(SICI)1099-1514(199905/06)20:3%3c127::AID-OCA650%3e3.0.CO;2-I
Kim BH, Ikeda T, Park HS (1999) Electrochemical activity of an Fe ( III ) -reducing bacterium, Shewanella putrefaciens IR-1, in the presence of alternative electron acceptors. Biotechnol Tech 200:475–478. https://doi.org/10.1023/A:1008993029309
Marsili E, Baron DB, Shikhare ID (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci U S A 105:3968–3973. https://doi.org/10.1073/pnas.0710525105
Call DF, Logan BE (2011) A method for high throughput bioelectrochemical research based on small scale microbial electrolysis cells. Biosens Bioelectron 26:4526–4531. https://doi.org/10.1016/j.bios.2011.05.014
Harrison JG, Griffin EA (2020) The diversity and distribution of endophytes across biomes, plant phylogeny and host tissues: how far have we come and where do we go from here? Environ Microbiol 22:2107–2123. https://doi.org/10.1111/1462-2920.14968
Mao L, Verwoerd WS (2013) Selection of organisms for systems biology study of microbial electricity generation: a review. Int J Energy Environ Eng 4:1–18. https://doi.org/10.1186/2251-6832-4-17
Zekker I, Bhowmick GD, Priks H (2020) ANAMMOX-denitrification biomass in microbial fuel cell to enhance the electricity generation and nitrogen removal efficienc. Biodegradation 31:249–264. https://doi.org/10.1007/s10532-020-09907-w
Jssa B, Raunija TSK, Verma A (2021) Impregnated thermoset pre-pressurized carbon composite electrodes in microbial fuel cell: Compositional functionalities influence on ORR with reference to graphite. Fuel 285:119273. https://doi.org/10.1016/j.fuel.2020.119273
Megaw J, Gilmore BF (2016) Draft genome sequence of Staphylococcus succinus strain CSM-77, a moderately halophilic bacterium isolated from a Triassic salt mine. Genome Announc 4:e00532-e616. https://doi.org/10.1128/genomeA.00532-16
Zhou H, Yao Z, Shi H (2017) Draft genome sequence of Staphylococcus succinus subsp. succinus type strain DSM 14617, isolated from plant and soil inclusions within 25- to 35-million-year-old Dominican amber. Genome Announc 5:e01521-e1616. https://doi.org/10.1128/genomeA.01521-16
Place RB, Hiestand D, Burri S (2002) Staphylococcus succinus subsp. casei subsp. nov., a dominant isolate from a surface ripened cheese. Syst Appl Microbiol 25:353–359. https://doi.org/10.1078/0723-2020-00130
Greppi A, Ferrocino I, La Storia A (2015) Monitoring of the microbiota of fermented sausages by culture independent rRNA-based approaches. Int J Food Microbiol 212:67–75. https://doi.org/10.1016/j.ijfoodmicro.2015.01.016
Gajaraj S, Hu Z (2014) Integration of microbial fuel cell techniques into activated sludge wastewater treatment processes to improve nitrogen removal and reduce sludge production. Chemosphere 117:151–157. https://doi.org/10.1016/j.chemosphere.2014.06.013
Zhang GD, Zhao QL, Jiao Y (2012) Efficient electricity generation from sewage sludge usingbiocathode microbial fuel cell. Water Res 46:43–52. https://doi.org/10.1016/j.watres.2011.10.036
Ling LJ, Yang CY, Li ZB (2021) Electrical performance and application in sewage treatment of an electricigenic strain Stenotrophomonas acidaminiphila EFS1. Microbiology China 48:1504–1513. https://doi.org/10.13344/j.microbiol.china.200820
Logan BE, Rossi R, Ragab A (2019) Electroactive microorganisms in bioelectrochemical systems. Nat Rev Microbiol 17:307–319. https://doi.org/10.1038/s41579-019-0173-x
Acknowledgements
We are grateful to Northwest Normal University for its cooperation with this study.
Funding
This work was supported in part by Lanzhou Science and Technology Plan Project 2018–1-104; Special funds for guiding the Development of Scientific and technological Innovation in Gansu Province 2019ZX-05; Industrial support Plan Project of Colleges and Universities in Gansu Province 2020C-21.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by HL, CY, ZL, and LL. The first draft of the manuscript was written by HL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
All authors clarify and agree to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ling, L., Luo, H., Li, Z. et al. Isolation, Identification and Characteristic Analysis of Plant Endophyte Electrogenic Bacteria Shinella zoogloeoides SHE10. Curr Microbiol 79, 268 (2022). https://doi.org/10.1007/s00284-022-02964-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02964-9