Abstract
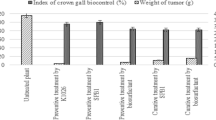
The present study was conducted to assess the biocontrol activity of biosurfactants obtained from Bacillus species A5F. The variables significantly influencing the production of biosurfactants under in vitro conditions were further optimized using response surface methodology. Optimal values of selected culture variables, i.e., glucose, soybean oil, and incubation time were 3.5 g l−1, 3.5 ml l−1, and 78 h, respectively, resulting in 2.14-fold enhancement in biosurfactant levels in 5 l fermentor. Identified biosurfactants had a significant effect on chlorophyll content, shoot biomass, number of pods, and seed weight. Biosurfactants also reduced the disease incidence in S. sclerotiorum infected soybean plants and showed antagonistic action against major phytopathogens by disrupting the hyphal cell wall. 16% reduction in ITS gene copy number was observed as compared to control with less non-target effect upon biosurfactant spray on foliar parts of soybean. Thus, the study confirms that biosurfactants from strain A5F can be used as a potent biocontrol agent to control sclerotium wilt on soybean plants.





Similar content being viewed by others
References
Pathak KV, Keharia H (2014) Application of extracellular lipopeptide biosurfactant produced by endophytic Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) in microbially enhanced oil recovery (MEOR). 3 Biotech 4(1):41–8
Almeida F, Rodrigues ML, Coelho C (2019) The still underestimated problem of fungal diseases worldwide. Front Microbiol 10:214
Banat IM, Satpute SK, Cameotra SS, Patil R, Nyayanit NV (2014) Cost effective technologies and renewable substrates for biosurfactants’ production. Front Microbiol 5:697
Wrather A, Shannon G, Balardin R, Carregal L, Escobar R, Gupta GK et al (2010) Effect of diseases on soybean yield in the top eight producing countries in 2006. Plant Health Prog 11(1):29
Cochrane SA, Vederas JC (2016) Lipopeptides from Bacillus and Paenibacillus spp.: a gold mine of antibiotic candidates. Med Res Rev 36(1):4–31
Reis RS, Pacheco GJ, Pereira AG, Freire DMG (2013) Biosurfactants: production and applications. Biodegradation—life of science. InTech, London, pp 31–61
Latha S, Sivaranjani G, Dhanasekaran D (2017) Response surface methodology: A non-conventional statistical tool to maximize the throughput of Streptomyces species biomass and their bioactive metabolites. Crit Rev Microbiol 43(5):567–582
Maget-Dana R, Peypoux F (1994) Iturins, a special class of pore-forming lipopeptides: biological and physicochemical properties. Toxicology 87(1–3):151–174
Deleu M, Bouffioux O, Razafindralambo H, Paquot M, Hbid C, Thonart P et al (2003) Interaction of surfactin with membranes: a computational approach. Langmuir 19(8):3377–3385
Wu S, Liu G, Zhou S, Sha Z, Sun C (2019) Characterization of antifungal lipopeptide biosurfactants produced by marine bacterium Bacillus sp. CS30. Mar Drugs 17(4):199
Honma M, Tanaka K, Konno K, Tsuge K, Okuno T, Hashimoto M (2012) Termination of the structural confusion between plipastatin A1 and fengycin IX. Bioorg Med Chem 20(12):3793–3798
Kumar A, Saini S, Wray V, Nimtz M, Prakash A, Johri BN (2012) Characterization of an antifungal compound produced by Bacillus sp. strain A5F that inhibits Sclerotinia sclerotiorum. J Basic Microbiol 52(6):670–8
Siegmund I, Wagner F (1991) New method for detecting rhamnolipids excreted byPseudomonas species during growth on mineral agar. Biotechnol Tech 5(4):265–268
Morikawa M, Hirata Y, Imanaka T (2000) A study on the structure–function relationship of lipopeptide biosurfactants. Biochim Biophys Acta Mol Cell Biol Lipids 1488(3):211–218
Cooper DG, Goldenberg BG (1987) Surface-active agents from two Bacillus species. Appl Environ Microbiol 53(2):224–229
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9(1):29–33
Zhang Y, Miller RM (1995) Effect of rhamnolipid (biosurfactant) structure on solubilization and biodegradation of n-alkanes. Appl Environ Microbiol 61(6):2247–2251
Mukherjee S, Das P, Sivapathasekaran C, Sen R (2009) Antimicrobial biosurfactants from marine Bacillus circulans: extracellular synthesis and purification. Lett Appl Microbiol 48(3):281–288
Ramarathnam R, Bo S, Chen Y, Fernando WD, Xuewen G, De Kievit T (2007) Molecular and biochemical detection of fengycin-and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Can J Microbiol 53(7):901–11
Nalini S, Parthasarathi R (2013) Biosurfactant production by Serratia rubidaea SNAU02 isolated from hydrocarbon contaminated soil and its physico-chemical characterization. Biores Technol 147:619–622
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat softw 25:1–18
Kassambara A, Mundt F (2020) Factoextra: extract and visualize the results of multivariate data analyses; R package version 1.0. 7. 2020. 2021
Wickham H, Hester J, Chang W (2019) devtools: tools to make developing R packages easier (R package version 2.0. 2)
Satpute SK, Banpurkar AG, Dhakephalkar PK, Banat IM, Chopade BA (2010) Methods for investigating biosurfactants and bioemulsifiers: a review. Crit Rev Biotechnol 30(2):127–144
Volchenko NN, Karasev SG, Nimchenko DV, Karaseva EV (2007) Cell hydrophobicity as a criterion of selection of bacterial producers of biosurfactants. Microbiology 76(1):112–114
Hošková M, Ježdík R, Schreiberová O, Chudoba J, Šír M, Čejková A et al (2015) Structural and physiochemical characterization of rhamnolipids produced by Acinetobacter calcoaceticus, Enterobacter asburiae and Pseudomonas aeruginosa in single strain and mixed cultures. J Biotechnol 193:45–51
Ferraz C, De Araújo ÁA, Pastore GM (2002) The influence of vegetable oils on biosurfactant production by Serratia marcescens. Appl Biochem Biotechnol 98(1):841–847
Zhou D, Hu F, Lin J, Wang W, Li S (2019) Genome and transcriptome analysis of Bacillus velezensis BS-37, an efficient surfactin producer from glycerol, in response to d-/l-leucine. Microbiol Open 8(8):e00794
Kim H-S, Jeon J-W, Kim B-H, Ahn C-Y, Oh H-M, Yoon B-D (2006) Extracellular production of a glycolipid biosurfactant, mannosylerythritol lipid, by Candida sp. SY16 using fed-batch fermentation. Appl Microbiol Biotechnol 70(4):391–396
Bajpai A, Singh B, Johri BN (2020) Rhamnolipids and siderophores from Pseudomonas protegens strain BNJ-SS-45 isolated from wheat rhizosphere. Environ Sustain 3:219–228
Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34(6):1037–1062
Chenniappan C, Narayanasamy M, Daniel GM, Ramaraj GB, Ponnusamy P, Sekar J, Ramalingam PV (2019) Biocontrol efficiency of native plant growth promoting rhizobacteria against rhizome rot disease of turmeric. Biol Control 129:55–64
Zhang Q-X, Zhang Y, Shan H-H, Tong Y-H, Chen X-J, Liu F-Q (2017) Isolation and identification of antifungal peptides from Bacillus amyloliquefaciens W10. Environ Sci Pol Res 24(32):25000–25009
Harwood CR, Mouillon JM, Pohl S, Arnau J (2018) Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol Rev 42(6):721–738
Kowall M, Vater J, Kluge B, Stein T, Franke P, Ziessow D (1998) Separation and characterization of surfactin isoforms produced by Bacillus subtilis OKB 105. J Colloid Interf Sci 204:1–8
Sharma D, Saharan BS, Chauhan N, Bansal A, Procha S (2014) Production and structural characterization of Lactobacillus helveticus derived biosurfactant. Sci World J 493548:1–9
Sarubbo LA, Maria da Gloria CS, Durval IJB, Bezerra KGO, Ribeiro BG, Silva IA, Banat IM (2022) Biosurfactants: production, properties, applications, trends, and general perspectives. Biochem Eng J 181:108377
Hernández-Morales A, Martínez-Peniche R-A, Arvizu-Gómez J, Arvizu-Medrano S-M, Rodríguez-Ontiveros A, Ramos-López M-A et al (2018) Production of a mixture of fengycins with surfactant and antifungal activities by Bacillus sp. MA04, a versatile PGPR. Ind J Microbiol 58(2):208–213
Chen L, Heng J, Qin S, Bian K (2018) A comprehensive understanding of the biocontrol potential of Bacillus velezensis LM2303 against Fusarium head blight. PLoS ONE 13(6):e0198560
Romero D, de Vicente A, Rakotoaly RH, Dufour SE, Veening J-W, Arrebola E et al (2007) The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol Plant Microbe Interact 20(4):430–440
Gong A-D, Li H-P, Yuan Q-S, Song X-S, Yao W, He W-J et al (2015) Antagonistic mechanism of iturin A and plipastatin A from Bacillus amyloliquefaciens S76–3 from wheat spikes against Fusarium graminearum. PLoS ONE 10(2):e0116871
Scotti-Campos P, Semedo JN, Pais IP, Oliveira M, Passarinho J, Santos M, Almeida AS, Costa AR, Pinheiro N, Bagorro C (2015) Physiological responses to drought in four developed Triticum aestivum groups. Emir J Food Agri 27:178–185
Mehetre GT, Dastager SG, Dharne MS (2019) Biodegradation of mixed polycyclic aromatic hydrocarbons by pure and mixed cultures of biosurfactant producing thermophilic and thermotolerant bacteria. Sci Total Environ 679:52–60
Malina A, Shai Y (2005) Conjugation of fatty acids with different lengths modulates the antibacterial and antifungal activity of a cationic biologically inactive peptide. Biochem J 390(3):695–702
Meena KR, Kanwar SS (2015) Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. BioMed Res Int. https://doi.org/10.1155/2015/473050
Acknowledgements
The authors wish to thanks National Academy of Sciences India, Allahabad for the grants given to BNJ and Jawaharlal Nehru Memorial Fund doctoral scholarship awarded to AB to carry out the research. Instrumentation facility received by DBT Builder Program and IIT Bombay, SAIF for LC-QToF-MS and SEM analysis are gratefully acknowledged.
Funding
This work was financially supported by the grants of National Academy of Sciences India, Allahabad (Grant No. NAS/201/7/2017-18) to BNJ and Jawaharlal Nehru Memorial Fund doctoral scholarship to AB.
Author information
Authors and Affiliations
Contributions
Manuscript writing, material preparation, data collection was performed by AB and statistical analysis was performed by RA. Proofreading of the manuscript was carried out by AP and BNJ. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bajpai, A., Agnihotri, R., Prakash, A. et al. Biosurfactant from Bacillus sp. A5F Reduces Disease Incidence of Sclerotinia sclerotiorum in Soybean Crop. Curr Microbiol 79, 206 (2022). https://doi.org/10.1007/s00284-022-02897-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02897-3