Abstract
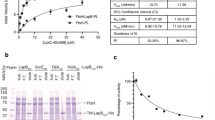
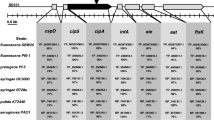
BglG, an RNA binding regulatory protein encoded by the β-glucoside (bgl) operon of E. coli is known to be involved in the regulation of several metabolic functions in stationary phase. A genome-wide comparative transcriptome analysis performed earlier between a ∆bglG strain and its isogenic WT counterpart revealed that genes involved in lipopolysaccharide (LPS) biosynthesis and transport were significantly down-regulated in the absence of BglG in stationary phase, suggesting a role for BglG in their regulation. We have investigated the involvement of BglG in LPS biosynthesis and transport. Consistent with the down-regulation of LPS synthesis and transport genes, the ∆bglG strain showed a loss of permeability barrier specifically in stationary phase, which could be rescued by introduction of wild type bglG on a plasmid. A search for a putative transcription factor involved in the regulation mediated by BglG led to the identification of GadE, which is one of the primary positive regulators of pH homeostasis and LPS core biosynthesis. Using RNA mobility shift and stability assays, we show that BglG binds specifically to gadE mRNA and enhances its stability. Consistent with this, loss of gadE leads to a partial defect in permeability. Based on our findings, we propose a model for the molecular mechanism involved in the regulation on LPS synthesis and transport by BglG.






Similar content being viewed by others
Abbreviations
- LPS:
-
Lipopolysaccharide
- PTS:
-
Phosphotransferase system
- RAT:
-
Ribonucleic antiterminator
- GAD:
-
Glutamate dependent
- RNA EMSA:
-
Ribonucleic acid electrophoretic mobility shift assay
References
Prasad I, Schaefler S (1974) Regulation of the β-glucoside system in Escherchia coli K-12. J Bacteriol 120(2):638–650
Schnetz K, Toloczyki C, Rak B (1987) Beta-glucoside (bgl) operon of Escherichia coli K-12: nucleotide sequence, genetic organization, and possible evolutionary relationship to regulatory components of two Bacillus subtilis genes. J Bacteriol 169(6):2579–2590
Mahadevan S, Reynolds A, Wright A (1987) Positive and negative regulation of the bgl operon in Escherichia coli. J Bacteriol 169(6):2570–2578
Mahadevan S, Wright A (1987) A bacterial gene involved in transcription antitermination: regulation at a rho-independent terminator in the bgl operon of E. coli. Cell 50(3):485–494
Schnetz K, Rak B (1988) Regulation of the bgl operon of Escherichia coli by transcriptional antitermination. EMBO J 7(10):3271–3277
Aymerich S, Steinmetz M (1992) Specificity determinants and structural features in the RNA target of the bacterial antiterminator proteins of the BglG/SacY family. Proc Natl Acad Sci USA 89(21):10410–10414
Houman F, Diaz-Torres MR, Wright A (1990) Transcriptional antitermination in the bgl operon of E. coli is modulated by a specific RNA binding protein. Cell 62(6):1153–1163
Amster-Choder O, Houman F, Wright A (1989) Protein phosphorylation regulates transcription of the β-glucoside utilization operon in E. coli. Cell 58(5):847–855
Amster-Choder O, Wright A (1990) Regulation of activity of a transcriptional anti-terminator in E. coli by phosphorylation in vivo. Science 249(4968):540–542
Amster-Choder O, Wright A (1992) Modulation of the dimerization of a transcriptional antiterminator protein by phosphorylation. Science 257(5075):1395–1398
Schnetz K, Rak B (1990) Beta-glucoside permease represses the bgl operon of Escherichia coli by phosphorylation of the antiterminator protein and also interacts with glucose-specific enzyme III, the key element in catabolite control. Proc Natl Acad Sci USA 87(13):5074–5078
Andersen C, Rak B, Benz R (1999) The gene bglH present in the bgl operon of Escherichia coli, responsible for uptake and fermentation of β-glucosides encodes for a carbohydrate-specific outer membrane porin. Mol Microbiol 31(2):499–510
Madan R, Kolter R, Mahadevan S (2005) Mutations that activate the silent bgl operon of Escherichia coli confer a growth advantage in stationary phase. J Bacteriol 187(23):7912–7917
Harwani D, Zangoui P, Mahadevan S (2012) The β-glucoside (bgl) operon of Escherichia coli is involved in the regulation of oppA, encoding an oligopeptide transporter. J Bacteriol 194(1):90–99
Shukla S, Mahadevan S (2019) The ridA gene of E. coli is indirectly regulated by BglG through the transcriptional regulator Lrp in stationary phase. Microbiology 165(6):683–696
Shukla S (2018) Investigations on the regulatory role of BglG on the expression of ridA and pleiotropic effects of bglG deletion in E. coli. PhD Thesis, Indian Institute of Science, Bangalore, India
Green MR, Sambrook J (2012) Molecular cloning: a laboratory manual. (Fourth Edition) Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97(12):6640–6645
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3(6):1101–1108
Hitchcock PJ, Brown TM (1983) Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol 154(1):269–277
De Biase D, Tramonti A, Bossa F, Visca P (1999) The response to stationary-phase stress conditions in Escherichia coli: role and regulation of the glutamic acid decarboxylase system. Mol Microbiol 32(6):1198–1211
Coldham NG, Webber M, Woodward MJ, Piddock LJ (2010) A 96-well plate fluorescence assay for assessment of cellular permeability and active efflux in Salmonella enterica serovar Typhimurium and Escherichia coli. J Antimicrob Chemother 65(8):1655–1663
Whitfield C, Trent MS (2014) Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem 83:99–128
Klein G, Lindner B, Brabetz W, Brade H, Raina S (2009) Escherichia coli K-12 suppressor-free mutants lacking early glycosyltransferases and late acyltransferases: minimal lipopolysaccharide structure and induction of envelope stress response. J Biol Chem 284(23):15369–15389
Hommais F, Krin E, Coppee J-Y, Lacroix C, Yeramian E, Danchin A, Bertin P (2004) GadE (YhiE): a novel activator involved in the response to acid environment in Escherichia coli. Microbiology 150(1):61–72
Hommais F, Krin E, Laurent-Winter C, Soutourina O, Malpertuy A, Le Caer JP, Danchin A, Bertin P (2001) Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein. H-NS Mol Microbiol 40(1):20–36
Tucker DL, Tucker N, Conway T (2002) Gene expression profiling of the pH response in Escherichia coli. J Bacteriol 184(23):6551–6558
Masuda N, Church GM (2003) Regulatory network of acid resistance genes in Escherichia coli. Mol Microbiol 48(3):699–712
Gulati A, Mahadevan S (2001) The Escherichia coli antiterminator protein BglG stabilizes the 5′ region of the bgl mRNA. J Biosci 26(2):193–203
Madan R, Moorthy S, Mahadevan S (2008) Enhanced expression of the bgl operon of Escherichia coli in the stationary phase. FEMS Microbiol Lett 288(1):131–139
Lopilato J, Wright A (1990) Mechanisms of activation of the cryptic bgl operon of Escherichia coli K-12. In: Drlica K, Riley M (eds) The bacterial chromosome. American Society for Microbiology, Washington, pp 435–444
Khan MA, Isaacson RE (1998) In vivo expression of the β-glucoside (bgl) operon of Escherichia coli occurs in mouse liver. J Bacteriol 180(17):4746–4749
Vashishtha K, Mahadevan S (2020) Catabolism of aromatic β-glucosides by bacteria can lead to antibiotics resistance. Arch Microbiol 202(6):1301–1315
Zambrano MM, Siegele DA, Almiron M, Tormo A, Kolter R (1993) Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Sci 259(5102):1757–1760
Amann E, Ochs B, Abel KJ (1988) Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69(2):301–315
Acknowledgements
This study was funded by the Department of Biotechnology (DBT) through a partnership programme with the Indian Institute of Science. The authors are also grateful to the Department of Science and Technology (DST) and the Universities Grants Commission (UGC) for infrastructural support. KV and SS are recipients of research fellowship from the Institute. We also thank the two anonymous referees for their constructive comments.
Funding
This study was funded by the Department of Biotechnology (DBT), Government of India, through a partnership program with the Indian Institute of Science. Infrastructural support was also provided by the Department of Science and Technology (DST) and the Universities Grants Commission (UGC), Government of India.
Author information
Authors and Affiliations
Contributions
KV and SM designed the experiments, KV and SS carried out the experiments, and KV and SM wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in terms of the content of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vashishtha, K., Shukla, S. & Mahadevan, S. Involvement of BglG in Lipopolysaccharides (LPS) Synthesis and Transport in Stationary Phase in E. coli. Curr Microbiol 79, 153 (2022). https://doi.org/10.1007/s00284-022-02837-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02837-1