Abstract
The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is threatening public health. A large number of affected people need to be hospitalized. Immunocompromised patients and ICU-admitted patients are predisposed to further bacterial and fungal infections, making patient outcomes more critical. Among them, COVID-19-associated candidiasis is becoming more widely recognized as a part of severe COVID-19 sequelae. While the molecular pathophysiology is not fully understood, some factors, including a compromised immune system, iron and zinc deficiencies, and nosocomial and iatrogenic transmissions, predispose COVID-19 patients to candidiasis. In this review, we discuss the existing knowledge of the virulence characteristics of Candida spp. and summarize the key concepts in the possible molecular pathogenesis. We analyze the predisposing factors that make COVID-19 patients more susceptible to candidiasis and the preventive measures which will provide valuable insights to guide the effective prevention of candidiasis in COVID-19 patients.
Similar content being viewed by others
Introduction
The novel coronavirus, SARS-CoV-2, first broke out in Wuhan, China, in December 2019 and has since spread worldwide, resulting in over 379 million confirmed cases and over 5.6 million deaths (till Feb 02, 2022) [1]. COVID-19 is caused by the SARS-CoV-2 and is characterized by mild-to-moderate respiratory illness, with symptoms, such as fever, coughing, fatigue, and breathing difficulties [2,3,4]. Individuals with underlying medical conditions or weakened immune systems are more likely to acquire the severe forms of COVID-19, in which the infection causes respiratory deterioration and may result in acute respiratory distress syndrome (ARDS) [3, 5]. Critically ill COVID-19 patients with ARDS, especially those admitted to the intensive care unit (ICU) and required mechanical ventilation, are more likely to develop nosocomial fungal co-infections [6,7,8,9]. Reports on the prevalence of bacterial and fungal co-infections and bacterial super-infection in COVID-19 patients have progressively increased worldwide [9,10,11,12,13]. A rare but lethal infection of mucormycosis (black fungus) and pulmonary aspergillosis has been reported in steroid treated and critically ill COVID-19 patients [14, 15]. COVID-19-associated mucormycosis, in particular, caused by mucorales such as Rhizopus arrhizus, Rhizomucor pusillus, Apophysomyces variabilis and, Lichtheimia corymbifera has been surging in India [16]. Although COVID-19–associated mucormycosis is the most common fungal infection in COVID-19 patients, candidiasis, a less common and less studied fungal infection among COVID-19 patients has emerged in several countries, including India [17, 18], Iran [19], China [6], UK [20] and is growing more prevalent.
Candidiasis is an opportunistic fungal infection caused by Candida species. Preventing candidiasis infections has long been a medical annoyance, and now COVID-19-associated candidiasis (CAC) is posing new challenges [21]. The precise pathogenesis of this fungal infection in COVID-19 patients is yet to be fully determined, and the knowledge on fungal co-infections is limited. As a result, incomplete understanding of the pathogenesis of candidiasis, lack of understanding of the possible risk factors that predispose COVID-19 patients to candidiasis, as well as lack of proper preventive measures may lead to misdiagnosis of secondary candidiasis in COVID-19 patients, worsening COVID-19 outcomes. Therefore, in this review, we briefly discuss the possible underlying patho-mechanisms of CAC, predisposing factors, and treatment strategies. Taken together, this review will add to our current knowledge of the proper management of CAC patients.
Candidiasis and Its Causative Agents
Belonging to the Ascomycota phylum, the Candida genus comprises approximately 200 species, among which only 30 Candida species have been identified as human pathogens [22]. Candida spp. is a part of the normal human microbiota, inhabits the mucosal surfaces of gastrointestinal, respiratory, and genitourinary tracts, as well as on the skin [23]. Like other natural flora, Candida spp. has been associated with humans as harmless commensals and has co-evolved to maintain homeostasis and a symbiotic relationship with the host [24].
Fungi comprise a small proportion of the wide variety of microorganisms found in residential microbiota, where the presence of other microbes naturally suppresses their growth. The interactions between all the microbial populations involved and the interplay between the commensal microbiome and the host immunity dictate whether a particular species will be virulent toward the host or remain benign. For example, in the intestinal and female reproductive systems, lactic acid bacteria, also co-existing with Candida albicans, compete with C. albicans for adhesion sites and prevent fungal attachment and Candida virulence [25]. On the other hand, studies have also shown that different oral bacteria can facilitate C. albicans pathogenicity by adhering to C. albicans and forming complex communities known as multispecies biofilms [26]. Mostly, when a disruption in the balance between commensal bacteria and the host immune system occurs, it triggers the switch from the harmless to virulent state, causing overgrowth of Candida spp. and candidiasis. Therefore, although Candida spp. live as harmless commensals in healthy individuals, they have virulence features that exploit opportunities, such as a weakened host immune system to cause infections, making them opportunistic fungi.
As an opportunistic pathogen, Candida spp. can cause two major types of infections in humans: superficial infections (which include cutaneous and mucosal candidiasis) and invasive systemic infections [23]. Superficial candidiasis arises from saprophytic Candida spp. blastospores residing in the normal flora that affect skin, gastrointestinal, vaginal, esophageal, and oropharyngeal mucosa and form white lesions or patches [27]. Superficial infections are so frequent that 75% of women are affected at least once in their lifetime by Vulvovaginal candidiasis (VVC) and about 90% of human immunodeficiency virus (HIV)-positive patients suffer from Oropharyngeal candidiasis (OPC) [28]. In an immunocompromised host, superficial candidiasis can overcome the weak host immunity and spread in the bloodstream, evolving into invasive systemic candidiasis called candidemia and can eventually colonize vital organs, causing disseminated candidemia. This bloodstream fungal infection can also be acquired via invasive medical devices, such as catheters [29]. Although less frequent than superficial candidiasis, the systemic infection has a mortality rate of 35–80% [30, 31], even with first-line antifungal therapy [32]. In the USA, the overall cost of treating candidiasis is $1.7 billion per year, making it the most expensive of systemic fungal infections [33]. While C. albicans have gained notoriety by being the predominant Candida spp. recovered from candidemia and invasive candidiasis isolates, non-albicans Candida spp. are now also emerging as opportunistic pathogens [34, 35]. Particularly, Candida auris, an emerging multi-drug-resistant fungal pathogen, is a non-albican Candida spp. that spreads rapidly in critically ill patients, causing nosocomial infection outbreaks and is associated with high mortality rates [36].
COVID-19-Associated Candidiasis
Prevalence of CAC and Causal Isolates
Prevalence of CAC and infection rate was found to vary among different countries [12, 37,38,39,40,41,42] (Table 1). A case study in Iran found 5% of patients developed OPC; 70.7% of the isolates were found to be C. albicans, while the least prevalent species was C. tropicalis [19]. Studies in Egypt found cases of CAC in Gharbia and Cairo where four patients were diagnosed with oral and/or vaginal CAC [12, 38]. Another case study in Iran reflected greater rates of Candidemia, with 85.7% of fungal infections confirmed to be caused by C. albicans and C. glabrata and 14.3% caused by non-Candida spp. [37]. The study found that patients with CAC had a mortality rate of 100% even after treatment with antifungal drugs. A study in Spain found cases of candiduria, candidemia, and intra-abdominal candidiasis (IAC) [42], 0.7% of patients had fungal infections, 57.1% of which was caused due to infection with C. albicans. Patients with CAC had a mortality rate of 50%. A case study in Italy found 6.9% of patients developed candidemia upon receiving parenteral nutrition, ICU support, and antibiotic treatment [39]. The occurrence of candidemia and candiduria in a COVID-19 unit in Florida, USA, prompted a point prevalence survey, in which 35 patients were found to be colonized with C. auris, 8 of whom died within 30 days of screening [40]. C. auris is a particular species of concern that is associated with high mortality rates, atypical fomite spread, and multi-drug resistance; numerous cases of C. auris co-infections have been documented worldwide [43]. Even so, the extent of association of CAC with the mortality rate is unknown as most patients had comorbidities, like diabetes, chronic kidney disease, or cancer. A study in the UK found 12.6% of fungal infections caused in a cohort of 135 patients, among which 93.8% were candidemia due to C. albicans and C. parapsilosis [41]. The mortality rate for patients with fungal co-infections was 53%, much higher than the mortality rate in patients without fungal co-infections at 31%.
Symptoms of CAC
CDC recommends home isolation for COVID-19 or suspected COVID-19 patients in regards to the general population [44]. Since some common signs of CAC and COVID-19 overlap, they may not be distinguishable to the general population, thereby preventing an early diagnosis of CAC. Clinical characteristics of oral candidiasis (OC) are often yellow–white plaques or pseudomembranous structures found on surfaces of lips, intraoral mucosal layer, buccal mucosa, the palate, mouth floor, tongue, and/or oropharynx [12, 19, 38]. White lesions and reddened surfaces may also be found on the oral mucosa. The white plaques can be easily removed with gauze to reveal a reddened, non-ulcerated mucosa—a characteristic of OPC [45]. Most patients are asymptomatic—symptoms shown in severe cases due to these plaques or lesions are sensitivity, oral burning, dysphagia (difficulty in swallowing), glossitis, and/or glossagia [12, 19, 38, 45].
Some common symptoms of COVID-19 are the loss of taste [12, 38], dysphagia [38], mild-to-severe oral discomfort/burning [12, 38], and/or intermittent fevers [12, 38]. These are also symptoms of OPC [12, 19, 38]; Candidemia causes intermittent fevers and chills [46]. The overlapping symptoms may prevent the early diagnosis of CAC. Additionally, COVID-19 is associated with xerostomia or a dry mouth, leading to dysphagia [38]. Dysphagia is also a symptom of OPC [19, 38], thus making it difficult to distinguish between the two infections [12, 19, 38] by the untrained eye.
Virulence Factors and Candida Pathogenesis in Human Body
The molecular pathogenesis of Candida sp. is complex composed of different pathways, which are summarized in Fig. 1. Initial events of superficial candidiasis involve attachment of Candida spp. in the planktonic form to host surfaces followed by colonization at epithelial surfaces. Specialized proteins called adhesins are expressed in Candida spp. Upon the initial contact, which allows adherence to the host membranes and abiotic surfaces [51]. Upon contact with a surface, thigmotropism (contact sensing) and various environmental cues trigger the yeast-to-hypha transition, further guided by thigmotropism to penetrate deeper into epithelia [52]. Most Candida spp. is dimorphic, i.e., it can grow both as yeast and filamentous forms. However, some Candida spp., such as C. albicans, exhibit polymorphism by transitioning between ovoid-shaped yeast, pseudohyphae, and true hyphae forms [53]. Environmental stimuli, such as temperature change, nutrient starvation, oxidative stress, osmotic pressure, and pH stress, induce the heat shock proteins (HSPs) and adaptation pathways that regulate morphology-associated genes leading to filamentous growth and biofilm formation [52, 54]. The phenotype switching of Candida spp. predominantly from white to opaque, provides mating competency and offers adaptive advantage and host immune system evasion [55]. Candida spp. utilizes two routes of invasion: induced endocytosis and active penetration. The interaction of the invasin expressed on the fungal cell surface with the host epithelial cell receptors (E-cadherin on epithelial cells, N-cadherin on endothelial cells) triggers induced endocytosis, resulting in the engulfment of the fungi into the host cell [56, 57]. On the other hand, active penetration is mediated by means of hyphae extension and secretion of fungal hydrolases (proteases, phospholipases, and lipases) to disrupt host tissues, causing deep-seated infection [56]. An important virulence feature of Candida spp. is the formation of biofilm, which involves the attachment of yeast cells to biotic (host cells) and abiotic (e.g., catheters, dentures) surfaces, proliferation, accumulation of extracellular matrix material (ECM), and finally, dispersal of yeast cells from the mature biofilm that can establish new biofilms [57]. The ECM acts as a protective barrier, enhancing adhesion, hyphae production, colonization, and fungal invasion while protecting the cell from immunological attack, preventing ROS formation, and increasing resistance to antifungal agents [59, 60]. Finally, when Candida spp. escapes the immune system, penetrates vascular tissues, and enters the bloodstream, it can infect other tissues and essential organs, resulting in disseminated candidiasis.
Molecular pathogenesis of Candida spp. (1 and 2) Planktonic Candida spp. cells express adhesins that facilitate attachment to host cell surfaces. (3) Environmental stimuli and induce morphology-associated genes and thigmotropism stimulate the transition from yeast-to-hypha transition and hyphal-directed growth. (4) Some Candida spp. exhibit phenotype switching, i.e., the epigenetic switching from white to opaque cells. (5) The two routes of Candida spp. invasion are as follows: (5A) Induced endocytosis where the fungal cells are engulfed by the host cell and (5B) Active penetration where the fungal hydrolases mediate host tissue penetration. (6) Some Candida spp. form biofilms upon the attachment to both biotic and abiotic surfaces. When Candida spp. enters the bloodstream, they are disseminated to vital organs, causing disseminated candidiasis. The genes involved in virulence are listed along with the corresponding steps in the pathogenesis
Factors that Predispose Candidiasis in COVID-19 Patients
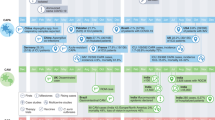
Candida spp. infections in COVID-19 patients could affect patient outcomes and complicate treatment efforts. There are various factors associated with COVID-19 that predispose patients to fungal infections (Fig. 2).
ICU
A combination of treatment-associated factors such as prolonged stays at the hospital or ICU, medical interventions, such as the use of mechanical ventilators, and intravenous catheters largely increase the risks of candidiasis [61]. COVID-19 patients with ARDS, who required admission into ICU had a greatly increased risk of infection through iatrogenic and/or nosocomial conditions [62, 63]. According to CDC, 21% and 12.1% of hospitalized patients aged over 65 required ICU admission and invasive mechanical ventilation respectively between January and June 2021 [64]. Moreover, patients at the ICU often require central venous catheterization and parenteral nutrition, while patients who do not require ventilation receive peripheral venous catheters [65, 66]. These medical devices penetrate the skin barrier and provide a direct path into the host interior [61, 67]. Some Candida spp. can form biofilms on these devices; the biofilms act as physical barriers that protect Candida spp. from antifungal treatment and the host’s immune defenses that are already weakened due to COVID-19 [61, 68]. Several studies demonstrated a strong positive correlation between length of ICU stay prior to infection and the development of infection, confirming that increased hospital and ICU stay increases the risk of co-infections [19, 26]. This may be due to an increased probability of fungal infections in a longer time frame and a gradually deteriorating immune system.
Antibiotics
The prolonged use of broad-spectrum antibiotics is also related to the disruption of the normal microbiota balance that switches Candida spp. from commensal to pathogenic morphogenesis [18, 19]. COVID-19 patients are often susceptible to bacterial co-infections for which antibiotics are prescribed, most commonly ceftriaxone and azithromycin [69]. A study found that 67% of COVID-19 patients require antibiotic use [69]. This has been shown to cause dysbiosis in local flora, allowing Candida spp. to outcompete other organisms to colonize and disseminate [19, 55, 61, 70]. Although the precise causes of dysbiosis are unknown, it may be caused by numerous other factors as well, such as the prescription of analgesics and the onset of lymphopenia during COVID-19 [71]. The level of dysbiosis may also differ depending on age, BMI, presence of comorbidities, etc. which also needs more exploration. A recent study found a direct correlation between the use of antibiotics and corticosteroids by a COVID-19 patient and the development of candidiasis [12, 72].
Corticosteroid
COVID-19 patients with ARDS are at risk of a severe inflammatory response known as the cytokine storm [72]. Immunosuppressive drugs like systemic or inhaled corticosteroids are often used as a treatment for this [72]. While this reduces the risk of hyperinflammatory response in the patients, it predisposes them to opportunistic fungal co-infections as fungal hyphae are protected from phagocytic attack [73, 74]. A study showed that corticosteroid treatment is linked to a 3.33-fold increase in the development of fungal co-infections [72]. The inhaled steroid fluticasone has been demonstrated to predispose patients to oral candidiasis through immune suppression [72, 75]. In addition to immunosuppression supporting opportunistic fungal growth, corticosteroids have also demonstrated inciting a hyperglycemic effect that predisposes patients to vulvovaginal candidiasis (VVC) [75, 76]. The increase in glucose levels creates a favorable environment for the proliferation of Candida spp. in the vagina; it was demonstrated that patients with a history of chronic corticosteroid use had a VVC recurrent rate of 65.9%, as opposed to non-users with a rate of 40.4% [75, 76]. Conclusively, corticosteroids have a high chance of predisposing patients with COVID-19 to candidiasis, especially in combination with other immunosuppressive drugs.
Weakened Immune System
It was observed that COVID-19 patients encountered a decrease in CD4 and CD8 T-cell count [68, 77, 78]. This may contribute to the development of lymphopenia observed in severe COVID-19 cases [78, 79]. Lymphopenia increases susceptibility to fungal attack due to an impaired immune system being unable to defend the host against infection. Subsequently, the already weakened immune system is further suppressed by drugs prescribed during COVID-19 infection—this may facilitate Candida spp. to manipulate and evade the host immune system [71, 73, 80]. COVID-19 patients with poor clinical outcomes or severe cases were found to have elevated levels of blood lactate [81] and increased levels of acid in blood and body tissues, i.e., acidosis [82]. When Candida spp. land on host niches rich in carboxylic acids and lactate, they assimilate these nutrients in order to modulate their cell wall architecture and mask the β-glucans on their cell wall [31, 83]. The masking of β-glucans, a key PAMP and a major fungal cell wall polysaccharide, aid Candida spp. which evade the host immune system. A study showed that low CD4 + counts were correlated with C. Neoformans infection [61]. Candida spp. are able to form biofilms [58] that play a key role in their virulence. It has been observed that a host defense against biofilms is through neutrophil cell death by DNA expulsion. The death of neutrophils cause the subsequent release of granulocytes that can potentially destroy fungal cells [67]. However, no such effect has been observed for Candida spp. infections. It has also been observed that leukocytes are unable to phagocytize biofilm-associated cells efficiently [67]. This may be why Candida spp. infections persist in COVID-19 patients experiencing a cytokine storm. Many COVID-19 patients experience a hyperinflammatory response or cytokine storm in which macrophage cell count increases significantly [84]. This should lead to increased phagocytosis and by extension, decreased fungal proliferation; however that is not the case in CAC. This may be collectively due to the decreased T-cell count and inefficient phagocytosis. Cytokine storms also increase pro-inflammatory cytokines, such as Tumor necrosis factor-α (TNF-α). TNF has been found to limit biofilm development in an in vitro C. albicans biofilm model, while TNF inhibition has been shown to promote biofilm metabolic activity [85]. The COVID-19 infection triggers a cytokine storm which leads to tissue damage and necrosis at the site of infection [78]. Due to this, COVID-19 patients are often prescribed TNF-α inhibitors [61]. Hence, the use of TNF-α inhibitors during COVID-19 may be a predisposing factor to the development of CAC. There are conflicting results associated with the role of macrophage levels and their effect on Candida spp. infections. A study found that reduced macrophage levels did not exacerbate infection by Candida spp. in the gut of mouse models as expected [70]. The effect of macrophage levels on fungal infection and its mechanism remains to be elucidated. Additionally, most cases of Candida spp. infections were found to occur due to nosocomial factors rather than immune-related ones. The significance of the sole effects of the immune response on fungal infections needs further investigation.
Iron Deficiency
COVID-19 patients develop dysregulations of iron homeostasis due to inflammation. This is characterized by high ferritin levels, referred to as hyper-ferritinemia, and decreased levels of circulating iron in the blood [86]. A study found over 80% of COVID-19 patients to have imbalanced levels of iron [86]. Hyper-ferritinemia has been linked to organ damage and ferroptosis, which increase the susceptibility of patients with COVID-19 to fungal co-infections due to the use of TNF-α antagonists [61, 78, 86]. It was observed in a study that patients with iron deficiency had a high prevalence of OC [87].
Iron acquisition mechanisms used by C. albicans include a high-affinity reductive system, a siderophore uptake system, and a heme–iron uptake system [88]. The reductive system obtains iron from ferritin, transferrin, as well as free iron from the environment reduces the ferric iron extracted to the ferrous form and transports it into the cell via a ferrous transporter complex [88]. It uses the invasin Als as the receptor for binding to ferritin, the predominant intracellular storage protein for iron [89]. In an iron-deficient stress state, Candida spp. secrete high-affinity iron chelators called siderophores that have a much higher affinity for iron than that of the host iron-binding proteins and thus are able to strip iron from host proteins [90]. Since COVID-19 patients have elevated levels of ferritin, the C. albicans reductive system can obtain more iron from higher ferritin sources, suggesting they may be at higher risks of co-infection [86, 90]. COVID-19 patients also have lowered blood pH due to increased pCO2 levels [91]. Ferritin is unstable at low pH and tends to release its protein-bound iron which can then be taken up by Candida spp. [87]. The elevated levels of ferritin together with low blood pH in COVID-19 patients may increase its proliferation and exacerbate the infection. A study also found that 30% of patients are susceptible to iron deficiencies after 2 months of COVID-19 onset, meaning COVID-19 patients are at risk of co-infection for a prolonged time period after disease onset [86]. Finally, COVID-19 has been associated with decreased electrolyte levels [92, 93]. Another study found that 16.3%, 12.5%, and 11.9% of patients had hyponatremia, hypokalemia, and hypochloremia, respectively [94]. C. albicans secrete hemolysins to lyse erythrocytes and uptakes the released hemoglobin via the receptors RBT5, RBT51, CSA1, CSA2, and PGA7 (RBT6) to extract iron from the heme group [90]. It has been shown that the hemolytic activity of Candida spp. is reduced by the presence of electrolytes [95]. Since electrolytes suppress the hemolytic activity of Candida spp., these lowered levels may possibly be linked to increased susceptibility in COVID-19 patients to co-infection [96].
Zinc Deficiency
Patients with COVID-19 were found to have significantly low levels of zinc [97]. Due to this, zinc supplements are a common prescription in COVID-19 cases [92]. Analogous to the siderophore uptake system for iron acquisition, C. albicans uses a “zincophore” zinc scavenger that is able to chelate zinc. The zincophores are the zinc-binding protein Pra1 (pH-regulated antigen 1) which binds to extracellular zinc and then re-associates with the fungal cell, allowing the fungal cell to uptake host zinc [98]. Since zinc deficiencies hinder fungal growth by inducing oxidative stress and inhibiting the activity of zincophores [99], this may increase the risk of COVID-19 patients receiving zinc supplements to fungal co-infections. However, conflicting studies showed that C. albicans are able to revert to a specific cell morphology with higher pathogenicity under zinc-starved conditions, suggesting that zinc may not be a large predisposing factor [94, 99].
Prevention and Treatment
Despite advancing therapeutic strategies, candidiasis continues to be a rising concern, particularly during the COVID-19 pandemic. The understanding of the spread of the disease helps shed light on preventive measures that reduce the risk of infection. Subsequently, diagnosis of candidiasis is crucial for species identification, which is required for administering further treatment plans. The currently available treatment option for candidiasis is limited with the persistence of antifungal resistance.
Preventive Measures
The majority of candidemia occurred due to nosocomial and/or iatrogenic conditions rather than reasons associated with immune suppression [80]. This suggests that the primary focus to prevent fungal co-infection should be related to medical interventions. The surge of global COVID-19 cases has resulted in a shortage of PPE [92, 100] and decreased practicing of routine health guidelines, such as proper hand hygiene [101]. An increased focus on ensuring strict health guidelines like controlled antimicrobial use, isolating patients infected with multi-drug resistant organisms, use of aseptic techniques, and the conduction of regular microbiological tests on COVID-19 patients may contribute to the prevention of co-infections [100, 101]. Healthcare professionals in the COVID-19 unit must use eye protection, gloves, gown, and must remove all PPE and perform hand hygiene before exiting the unit. Mobile computers and medical equipment must always be disinfected between uses. Moreover, infected catheters must be removed in the absence of alternative treatments, such as antifungal lock therapy [86]. Furthermore, a rapid diagnosis of CAC is required to prevent further fungal transmission to non-infected individuals and enable timely treatment of the infected patient to prevent poor prognosis.
Diagnosis Methods
Early diagnosis and treatment of Candidiasis are key factors in decreasing mortality rates in patients with co-infections. Initially, patients’ medical records are checked as standard protocol to see if there is a predisposition to CAC associated with medications, previous long duration of hospital stay, use of corticosteroids, etc. [19, 42].
Non-Sterile Culture
OC can be diagnosed by isolating and then identifying the Candida spp. [102]. The suspected sample is taken from oral tissues using a swab or oral rinse depending on the type of plaques or lesions present. In case of imperceptible lesions, an oral rinse or sputum collection is preferred. The samples are usually cultured on Saboraus Dextrose Agar (SDA) and incubated for 24 to 48 h at 37 °C [102]. Microbial culture is usually effective in diagnosing acute forms of OC. OPC was successfully diagnosed in COVID-19 patients using this method in a study conducted in Iran [19].
Respiratory cultures are done with samples, such as bronchoalveolar lavage (BAL), tissue biopsies, or cytology specimens [17, 103]. These undergo microscopy or histopathological examinations using fungal-specific staining techniques or fluorescent markers [103].
Sterile Culture
Cultures from sterile sites such as blood, peritoneal fluid, or pleural fluid are considered the reference standard for the diagnosis of invasive candidiasis [103]. Peripheral or Central Venous (CV) blood cultures are usually carried out to check for the growth of Candida spp. [104]. However, this method effectively diagnoses 50% of invasive candidiasis cases as the number of yeast cells available in the blood culture is low [62].
β-D-Glucan (BDG)
BDG testing is a non-culture method of diagnosis that depends on antigen/antibody assaying (known as Fungitell assay) [103]. 1–3 β-D-glucan (BDG) is a common fungal cell wall polysaccharide—so this method can detect a wide range of fungal species [103]. Since the method is more sensitive than blood culturing techniques, it is often used for the diagnosis of CAC [62]. It is effective, with a sensitivity and specificity of 80% [65]. This can be further improved with sequencing positive BDG results [103]. However, BDG testing is not widely available and may not be available to developing countries during the COVID-19 pandemic, making the diagnosis of CAC difficult in specific regions [41].
Molecular-Based Methods
Candida mannan antigens in serum samples can be detected via Enzyme-linked immunosorbent assay (ELISA) kits to diagnose invasive candidiasis [62]. This method is associated with comparatively high specificity and sensitivity [62].
T2-Candida is a multiplex PCR technique that amplifies intervening transcribed spacer-2 (ITS2) regions and uses T2 magnetic resonance to detect and identify C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei [65, 103]. The drawback to this method is that the number of Candida spp. that can be identified is limited [103]. An early diagnosis is especially important in the case of C. auris, an increasingly proliferating species. This is because C. auris is often misidentified using conventional diagnostic methods based on biochemistry or phenotype and requires specific molecular testing methods, such as sequencing and internal transcribed spacers [43]. 16 s rRNA sequencing is a high throughput, next-generation sequencing method that is also used for the identification of Candida spp. [105, 106]. 16 s rRNA gene is highly conserved with several variable regions. PCR primers are designed based on the conserved regions for universal amplification; variable regions are used to distinguish between different species [105].
Treatment
The immediate start of treatment is imperative to treat any fungal disease, including candidiasis [62, 107]. The three main classes of antifungals prescribed for the treatment of candidiasis are azoles, polyenes, and echinocandins. The antifungal of choice is dependent on disease severity, presence of comorbidities, prior fungal exposure, etc. [103]. Additionally, antifungal susceptibility is an increasingly pressing concern associated with the use of antifungals [19, 103]. Although antifungal susceptibility or degree of fungal resistance can be predicted upon identification of species, antifungal susceptibility testing (AST) should be done prior to prescription of antifungals to optimize treatment of candidiasis without further aggravating antifungal resistance [103].
Azoles
Azoles inhibit fungal growth by blocking the ergosterol production pathway [108]. The resulting decreased levels of ergosterol and subsequent buildup of sterol intermediates in the cytoplasmic membrane cause hindered growth [108]. Some commonly prescribed azoles for candidiasis treatment are fluconazole, miconazole, and clotrimazole [38, 45]. Fluconazole is usually administered for 7–14 days at a 50–100 mg dose per day, while clotrimazole is prescribed 5 times a day for 14 days in the form of 10 mg oral pills for COVID-19 patients [45]. A headache and hepatotoxicity are experienced for over 10% and 4–23% patients, respectively, as side effects [103]. A study in Cairo, Egypt showed the successful treatment of a COVID-19 patient with OPC with the simultaneous use of miconazole (4 times a day) and fluconazole (3 times a day) [38]. No separate side effects to these drugs were observed in the COVID-19 patients.
Polyenes
Polyenes such as nystatin is an antifungal agent that destroys fungal cells [109]. The drug binds to ergosterol in the cell membrane to induce changes in its permeability—eventually resulting in cell death [109]. Nystatin does not cause serious side effects when ingested orally and is often the choice of treatment of OC [45]. A patient in Egypt was prescribed the topical antifungal nystatin for treatment of OC; the dosage was four times a day along with chlorhexidine (an antibacterial mouthwash) twice per day—resulting in the successful treatment of OC within 10 days of the treatment [38].
Echinocandins
Echinocandins—such as caspofungin and micafungin—are lipopeptides that initiate fungal cell death by blocking the transmembrane glucan synthase complex, resulting in the disruption of BDG synthesis [103]. These are the preferred choice of treatment over azoles and polyenes as they are more tolerated by Candida spp. compared to azoles and polyenes [17, 62, 103]. Echinocandins are generally associated with positive outcomes upon removal of the CVC (if present) in patients [62].
Future Directions
The COVID-19 pandemic has led to a rise in immune-compromised individuals and subsequently, cases of hospitalization [78, 110]. This puts patients at risk of developing co-infections, such as invasive candidiasis. The diagnosis of co-infection is difficult due to the lack of symptoms or the development of symptoms similar to COVID-19 [100]. Therefore, more emphasis on precise diagnostic methods such as microbiological examinations should be given, especially to make diagnosis more accessible to developing countries. Methods to track the dissemination of Candida spp. infections should be developed to understand and prevent fungal spread. Thus, more research is needed to elucidate the exact mechanism of each step of Candida spp. pathogenesis. This could lead to the identification of specific genes responsible for virulence, and the subsequent development of treatment working to inhibit gene expression. The use of iron chelators, such as hydroxypyridone antimycotic and ciclopirox olamine, has demonstrated positive outcomes against infection [92]. It was observed that there was no development of fungal resistance toward Ciclopirox olamine [92]. This suggests that treatment targeting the host’s micronutrient acquisition may be a potentially promising avenue of research. More research should be conducted on identifying substances that can inactivate or hinder biofilm formation. Subsequently, multi-drug-resistant Candida spp. remains a pressing problem associated with fungal co-infections. Further research is crucial to develop methods that inhibit antifungal-resistant mechanisms of Candida spp. and the expansion of our limited range of antifungal treatment.
The etiology of candidiasis and its pathogenesis and virulence factors are crucial to further understand its association and inclination toward COVID-19 patients. Additionally, more research should be conducted that follows currently available antifungal treatments in COVID-19 patients to get a better understanding of the drug combinations and side effects of treatment. Collectively, these will provide gateways to the development of new therapeutic antimicrobial treatment strategies as well as the management of co-infections. The implementation of awareness programs based on the mechanism and spread of fungal co-infections could create public health awareness and incite behavioral changes that lower disease prevalence. The emphasis on these areas of research will contribute toward a decrease in mortality and morbidity of COVID-19 patients with fungal co-infections and will prevent future Candida-related epidemics that will undoubtedly otherwise have disastrous consequences on public health.
Conclusion
Candidiasis, an opportunistic fungal infection, has become more prevalent as the world continues to tackle COVID-19. It is crucial to understand the pathogenesis and mechanism of virulence to shed light on disease progression, especially in the case of co-infections. The transition of Candida spp. from commensal to pathogen, the ability to switch morphology and form biofilms, the progression of Candida spp. infection, and its virulence factors are not yet entirely elucidated. Additionally, the various highlighted factors that predispose COVID-19 patients to Candidiasis, their interactions, and individual effects must be well understood to prevent the development of opportunistic co-infections that drastically lower chances of survival. The paper also emphasizes possible methods of prevention and treatment with azoles, polyenes, and echinocandins. Conclusively, further research is required to curb the rapid increase of Candidiasis and lessen the burden of CAC in the world.
Data Availability
Not Applicable.
References
COVID Live - Coronavirus Statistics - Worldometer n.d. https://www.worldometers.info/coronavirus/ (accessed February 2, 2022).
Araf Y, Faruqui NA, Anwar S et al (2021) SARS-CoV-2: a new dimension to our understanding of coronaviruses. Int Microbiol 24:19–24. https://doi.org/10.1007/s10123-020-00152-y
Tabassum T, Rahman A, Araf Y, Ullah MA, Hosen MJ (2021) Prospective selected biomarkers in COVID-19 diagnosis and treatment. Biomark Med 15(15):1435–1449
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720
Goh KJ, Choong MC, Cheong EH, Kalimuddin S, Duu Wen S, Phua GC et al (2020) Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from coronavirus disease 2019 (COVID-19) infection. Ann Acad Med Singap 49(3):108–118
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223):507–513
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H et al (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8(5):475–481
Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R et al (2020) COVID-19 Associated Pulmonary Aspergillosis (CAPA)-from immunology to treatment. J Fungi (Basel). 6(2):91
Silva LN, de Mello TP, de Souza RL, Branquinha MH, Roudbary M, Dos Santos ALS (2020) Fungal infections in COVID-19-positive patients: a lack of optimal treatment options. Curr Top Med Chem 20(22):1951–1957
Rodriguez JY, Le Pape P, Lopez O, Esquea K, Labiosa AL, Alvarez-Moreno C (2020) Candida auris: a latent threat to critically ill patients with COVID-19. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1595
Bhagali R, Prabhudesai NP, Prabhudesai MN (2021) Post COVID-19 opportunistic candida retinitis: A case report. Indian J Ophthalmol 69(4):987–989
Riad A, Gad A, Hockova B, Klugar M (2020) Oral candidiasis in non-severe COVID-19 patients: call for antibiotic stewardship. Oral Surg. https://doi.org/10.1111/ors.12561
Didbaridze T, Ratiani L, Labadze N, Maziashvili T (2021) Prevalence and prognosis of candidiasis among Covid-19 patients: data from ICU department. Int J Prog Sci Technol 26(1):36–39
Lai C-C, Weng-Liang Yu (2021) COVID-19 associated with pulmonary aspergillosis: A literature review. J Microbiol Immunol Infection 54:46–53
Yusuf E, Seghers L, Hoek RAS, van den Akker JPC, Bode LGM, Rijnders BJA (2021) Aspergillus in critically Ill COVID-19 patients: a scoping review. J Clin Med 10:2469
Tabassum T, Araf Y, Moin AT et al (2022) COVID-19-associated-mucormycosis: possible role of free iron uptake and immunosuppression. Mol Biol Rep 49:747–754. https://doi.org/10.1007/s11033-021-06862-4
Kaur H, Singh D, Kajal NC. Rupali. Invasive candidiasis with cavitary lung lesion in a post-Covid-19 diabetic patient-A case report. J Clin Med Res. 2021;3(5):1–6.
Chowdhary A, Tarai B, Singh A, Sharma A (2020) Multidrug-resistant Candida auris infections in critically Ill coronavirus disease patients, India, April–July 2020. Emerg Infect Dis 26(11):2694–2696
Salehi M, Ahmadikia K, Mahmoudi S, Kalantari S, Jamalimoghadamsiahkali S, Izadi A, Kord M, Dehghan Manshadi SA, Seifi A, Ghiasvand F, Khajavirad N (2020) Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 63(8):771–778
Denny S, Abdolrasouli A, Elamin T, Gonzalo X, Charani E, Patel A, Donaldson H, Hughes S, Armstrong-James D, Moore LS, Mughal N (2021) A retrospective multicenter analysis of candidaemia among COVID-19 patients during the first UK pandemic wave. J Infect 82:276–316
Chen X, Liao B, Cheng L, Peng X, Xu X, Li Y et al (2020) The microbial co-infection in COVID-19. Appl Microbiol Biotechnol 104(18):7777–7785
Brandt ME, Lockhart SR (2012) Recent taxonomic developments with candida and other opportunistic yeasts. Curr Fungal Infect Rep 6(3):170–177
Niemiec MJ, Kapitan M, Polke M, Jacobsen ID. Commensal to pathogen transition of Candida albicans. Reference module in life sciences. Elsevier. 2017; 696–713.
Belkaid Y, Harrison OJ (2017) Homeostatic immunity and the microbiota. Immunity 46(4):562–576
Morales DK, Hogan DA (2010) Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog 6(4):e1000886
Montelongo-Jauregui D, Lopez-Ribot JL (2018) Candida interactions with the oral bacterial microbiota. J Fungi (Basel). 4(4):122
Fidel PL Jr, Wozniak KL (2010) Superficial candidiasis. Topley Wilson’s Microbiol Microbial Infect 4:255–272
Sawant B, Khan T (2017) Recent advances in delivery of antifungal agents for therapeutic management of candidiasis. Biomed Pharmacother 96:1478–1490
Kojic EM, Darouiche RO (2004) Candida infections of medical devices. Clin Microbiol Rev 17(2):255–267
Xiao Z, Wang Q, Zhu F, An Y (2019) Epidemiology, species distribution, antifungal susceptibility and mortality risk factors of candidemia among critically ill patients: a retrospective study from 2011 to 2017 in a teaching hospital in China. Antimicrob Resist Infect Control 8:89
Pfaller MA, Diekema DJ (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20(1):133–163
Raz-Pasteur A, Ullmann Y, Berdicevsky I (2011) The pathogenesis of Candida infections in a human skin model: scanning electron microscope observations. ISRN Dermatol. 2011:150642
Wilson LS, Reyes CM, Stolpman M, Speckman J, Allen K, Beney J (2002) The direct cost and incidence of systemic fungal infections. Value Health 5(1):26–34
Falagas ME, Roussos N, Vardakas KZ (2010) Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: a systematic review. Int J Infect Dis 14(11):e954–e966
Yapar N (2014) Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag 10:95–105
Ademe M, Girma F (2020) Candida auris: From multidrug resistance to pan-resistant strains. Infect Drug Res 13:1287
Arastehfar A, Shaban T, Zarrinfar H, Roudbary M, Ghazanfari M, Hedayati M-T et al (2021) Candidemia among iranian patients with severe covid-19 admitted to icus. J Fungi 7(4):280
Riad A, Gomaa E, Hockova B, Klugar M (2021) Oral candidiasis of COVID-19 patients: Case report and review of evidence. J Cosmet Dermatol 20(6):1580–1584
Antinori S, Bonazzetti C, Gubertini G, Capetti A, Pagani C, Morena V et al (2020) Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun Rev 19(7):102564
Prestel C, Anderson E, Forsberg K, Lyman M, de Perio MA, Kuhar D et al (2021) Candida auris outbreak in a covid-19 specialty care unit — florida, july–august 2020. MMWR Morb Mortal Wkly Rep 70(2):56–57
White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S et al (2020) A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. https://doi.org/10.2139/ssrn.3644400
Garcia-Vidal C, Sanjuan G, Moreno-Garcia E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M et al (2021) Incidence of co-infections and super-infections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect 27(1):83–88
Janniger EJ, Kapila R (2021) Public health issues with Candida auris in COVID-19 patients. World Med Health Policy. https://doi.org/10.1002/wmh3.472
CDC. Covid-19 and your health [Internet]. Centers for Disease Control and Prevention. 2020 [cited 2022 Feb 1]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/steps-when-sick.html.
Jerônimo LS, Lima RPE, Suzuki TYU, Discacciati JAC, Bhering CLB (2021) Oral candidiasis and covid-19 in users of removable dentures: is special oral care needed? GER 14:1–6
R AN, Rafiq NB. Candidiasis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 [cited 2021 Aug 13]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK560624/.
Shirvani F, Fattahi A (2021) Pulmonary candidiasis associated with COVID-19: evaluation of causative agents and their antifungal susceptibility patterns. Tanaffos 20(1):29
Al-Hatmi AMS, Mohsin J, Al-Huraizi A, Khamis F (2021) COVID-19 associated invasive candidiasis. J Infect 82:e45–e46. https://doi.org/10.1016/j.jinf.2020.08.005
Chowdhary A, Tarai B, Singh A, Sharma A (2020) Multidrug-Resistant Candida auris Infections in Critically Ill coronavirus disease patients, India, April–July 2020. Emerg Infect Dis J. https://doi.org/10.3201/eid2611.203504
de Almeida JN, Francisco EC, Hagen F, Brandão IB, Pereira FM, Presta Dias PH et al (2021) Emergence of Candida auris in Brazil in a COVID-19 intensive care unit. J Fungi 7:220. https://doi.org/10.3390/jof7030220
Liu Y, Filler SG (2011) Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot Cell 10(2):168–173
Cottier F, Muhlschlegel FA (2009) Sensing the environment: response of Candida albicans to the X factor. FEMS Microbiol Lett 295(1):1–9
Mukaremera L, Lee KK, Mora-Montes HM, Gow NAR (2017) Candida albicans Yeast, Pseudohyphal, and Hyphal Morphogenesis differentially affects immune recognition. Front Immunol 8:629
Gong Y, Li T, Yu C, Sun S (2017) Candida albicans Heat Shock Proteins and Hsps-associated signaling pathways as potential antifungal targets. Front Cell Infect Microbiol 7:520
Mba IE, Nweze EI (2020) Mechanism of Candida pathogenesis: revisiting the vital drivers. Eur J Clin Microbiol Infect Dis 39(10):1797–1819
Wachtler B, Citiulo F, Jablonowski N, Forster S, Dalle F, Schaller M et al (2012) Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS ONE 7(5):e36952
Phan QT, Fratti RA, Prasadarao NV, Edwards JE Jr, Filler SG (2005) N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J Biol Chem 280(11):10455–10461
Vila TV, Rozental S (2016) Biofilm formation as a pathogenicity factor of medically important fungi. Fungal Pathogenicity. 11:1–24
Cavalheiro M, Teixeira MC (2018) Candida biofilms: threats, challenges, and promising strategies. Front Med (Lausanne) 5:28
Hernandez-Chavez MJ, Perez-Garcia LA, Nino-Vega GA, Mora-Montes HM. Fungal Strategies to Evade the Host Immune Recognition. J Fungi (Basel). 2017;3(4).
Firacative C (2020) Invasive fungal disease in humans: are we aware of the real impact? Mem Inst Oswaldo Cruz 115:e200430
Arastehfar A, Carvalho A, Nguyen MH, Hedayati MT, Netea MG, Perlin DS et al (2020) Covid-19-associated candidiasis (Cac): an underestimated complication in the absence of immunological predispositions? JoF 6(4):211
Vincent J-L, Rello J, Marshall J, Silva E, Anzueto A, Martin CD et al (2009) International study of the prevalence and outcomes of infection in intensive care units. JAMA 302(21):2323–2329
Taylor CA. Severity of disease among adults hospitalized with laboratory-confirmed covid-19 before and during the period of sars-cov-2 b. 1. 617.(Delta) 2 predominance — covid-net, 14 states, january–august 2021. MMWR Morb Mortal Wkly Rep [Internet]. 2021 [cited 2022 Feb 1];70. Available from: https://www.cdc.gov/mmwr/volumes/70/wr/mm7043e1.htm.
Pittiruti M, Pinelli F, Pittiruti M, Pinelli F, Annetta MG, Bertoglio S et al (2020) Recommendations for the use of vascular access in the COVID-19 patients: an Italian perspective. Crit Care 24(1):269
Thibault R, Seguin P, Tamion F, Pichard C, Singer P (2020) Nutrition of the COVID-19 patient in the intensive care unit (Icu): a practical guidance. Crit Care 24(1):447
Tsui C, Kong EF, Jabra-Rizk MA (2016) Pathogenesis of Candida albicans biofilm. Pathogens Dis. https://doi.org/10.1093/femspd/ftw018
Song G, Liang G, Liu W (2020) Fungal co-infections associated with global covid-19 pandemic: a clinical and diagnostic perspective from china. Mycopathologia 185(4):599–606
Neto AGM, Lo KB, Wattoo A, Salacup G, Pelayo J, DeJoy R et al (2021) Bacterial infections and patterns of antibiotic use in patients with COVID-19. J Med Virol 93(3):1489–1495
Kobayashi-Sakamoto M, Tamai R, Isogai E, Kiyoura Y (2018) Gastrointestinal colonisation and systemic spread of Candida albicans in mice treated with antibiotics and prednisolone. Microb Pathog 117:191–199
Battaglini D, Robba C, Fedele A, Trancǎ S, Sukkar SG, Di Pilato V et al (2021) The role of dysbiosis in critically ill patients with covid-19 and acute respiratory distress syndrome. Front Med 8:671714
Segrelles-Calvo G, de S Araújo GR, Frases S. Systemic mycoses: a potential alert for complications in COVID-19 patients. Future Microbiology. 2020;15(14):1405–13.
Fraczek MG, Chishimba L, Niven RM, Bromley M, Simpson A, Smyth L et al (2018) Corticosteroid treatment is associated with increased filamentous fungal burden in allergic fungal disease. Journal of Allergy and Clinical Immunology 142(2):407–414
Ahmed MH, Hassan A (2020) Dexamethasone for the treatment of coronavirus disease (COVID-19): a review. SN Compr Clin Med. https://doi.org/10.1007/s42399-020-00610-8
Shenoy A, Gottlieb A. Probiotics for oral and vulvovaginal candidiasis: A review. Dermatologic Therapy. 2019 30;e12970.
Farhan MA, Moharram AM, Salah T, Shaaban OM (2019) Types of yeasts that cause vulvovaginal candidiasis in chronic users of corticosteroids. Med Mycol 57(6):681–687
Silva DL, Lima CM, Magalhaes VCR, Baltazar LM, Peres NTA, Caligiorne RB et al (2021) Fungal and bacterial co-infections increase mortality of severely ill COVID-19 patients. J Hosp Infect 113:145–154
Merad M, Martin JC (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20(6):355–362
Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S (2020) Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol 39(7):2085–2094
Davis SE, Hopke A, Minkin SC, Montedonico AE, Wheeler RT, Reynolds TB (2014) Masking of beta(1–3)-glucan in the cell wall of Candida albicans from detection by innate immune cells depends on phosphatidylserine. Infect Immun 82(10):4405–4413
Carpenè G, Onorato D, Nocini R, Fortunato G, Rizk JG, Henry BM et al (2021) Blood lactate concentration in COVID-19: a systematic literature review. Clin Chem Lab Med. https://doi.org/10.1515/cclm-2021-1115
Nechipurenko YD, Semyonov DA, Lavrinenko IA, Lagutkin DA, Generalov EA, Zaitceva AY et al (2021) The role of acidosis in the pathogenesis of severe forms of covid-19. Biology 10(9):852
Mayer FL, Wilson D, Hube B (2013) Candida albicans pathogenicity mechanisms. Virulence 4(2):119–128
Ombrello MJ, Schulert GS (2021) COVID-19 and cytokine storm syndrome: are there lessons from macrophage activation syndrome? Transl Res 232:1–12
Rocha FA, Alves AM, Rocha MF, de Aguiar CR, Brilhante RS, Pinto AC, de Melo NR, Girão VC, Sidrim JJ (2017) Tumor necrosis factor prevents Candida albicans biofilm formation. Sci Rep 7(1):1–7
Sonnweber T, Boehm A, Sahanic S, Pizzini A, Aichner M, Sonnweber B et al (2020) Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: a prospective observational cohort study. Respir Res 21(1):276
Fourie R, Kuloyo OO, Mochochoko BM, Albertyn J, Pohl CH (2018) Iron at the Centre of Candida albicans Interactions. Front Cell Infect Microbiol 8:185
Lewis RE, Lo HJ, Raad II, Kontoyiannis DP (2002) Lack of catheter infection by the efg1/efg1 cph1/cph1 double-null mutant, a Candida albicans strain that is defective in filamentous growth. Antimicrob Agents Chemother 46(4):1153–1155
Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR (1997) Nonfilamentous C. albicans mutants are avirulent. Cell 90(5):939–949
Fan Y, He H, Dong Y, Pan H (2013) Hyphae-specific genes HGC1, ALS3, HWP1, and ECE1 and relevant signaling pathways in Candida albicans. Mycopathologia 176(5–6):329–335
Elezagic D, Johannis W, Burst V, Klein F, Streichert T (2021) Venous blood gas analysis in patients with COVID-19 symptoms in the early assessment of virus positivity. J Laboratory Med 45(1):27–30
Oyagbemi AA, Ajibade TO, Aboua YG, Gbadamosi IT, Adedapo ADA, Aro AO et al (2021) Potential health benefits of zinc supplementation for the management of COVID-19 pandemic. J Food Biochem 45(2):e13604
De Carvalho H, Richard MC, Chouihed T, Goffinet N, Le Bastard Q, Freund Y et al (2021) Electrolyte imbalance in COVID-19 patients admitted to the Emergency Department: a case-control study. Intern Emerg Med. https://doi.org/10.1007/s11739-021-02632-z
Bedell GW, Soll DR (1979) Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun 26(1):348–354
Kumamoto CA, Vinces MD (2005) Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol 7(11):1546–1554
Malcok HK, Aktas E, Ayyildiz A, Yigit N, Yazgi H (2009) Hemolytic activities of the Candida species in liquid medium. Eurasian J Med 41(2):95
Jothimani D, Kailasam E, Danielraj S, Nallathambi B, Ramachandran H, Sekar P et al (2020) COVID-19: Poor outcomes in patients with zinc deficiency. Int J Infect Dis 100:343–349
Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Höfs S, Gratacap RL, Robbins J, Runglall M, Murciano C (2016) Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 532(7597):64–68
Malavia D, Lehtovirta-Morley LE, Alamir O, Weiß E, Gow NAR, Hube B et al (2017) Zinc limitation induces a hyper-adherent goliath phenotype in candida albicans. Front Microbiol 8:2238
Zhou P, Liu Z, Chen Y, Xiao Y, Huang X, Fan X-G. Bacterial and fungal infections in COVID-19 patients: A matter of concern. Infect Control Hosp Epidemiol. :1–2.
Rawson TM, Wilson RC, Holmes A (2021) Understanding the role of bacterial and fungal infection in COVID-19. Clin Microbiol Infect 27(1):9–11
Lewis MAO, Williams DW (2017) Diagnosis and management of oral candidosis. Br Dent J 223(9):675–681
Gonzalez-Lara MF, Ostrosky-Zeichner L (2020) Invasive candidiasis. Semin Respir Crit Care Med 41(01):003–012
Al-Hatmi AMS, Mohsin J, Al-Huraizi A, Khamis F (2021) COVID-19 associated invasive candidiasis. J Infect 82(2):e45–e46
Fuks G, Elgart M, Amir A, Zeisel A, Turnbaugh PJ, Soen Y et al (2018) Combining 16S rRNA gene variable regions enables high-resolution microbial community profiling. Microbiome 6(1):17
Sokol H, Leducq V, Aschard H, Pham H-P, Jegou S, Landman C et al (2017) Fungal microbiota dysbiosis in IBD. Gut 66(6):1039–1048
Talento AF, Hoenigl M (2020) Fungal infections complicating covid-19: with the rain comes the spores. Journal of Fungi 6(4):279
Shirvani F, Fattahi A (2021) Pulmonary candidiasis associated with COVID-19: Evaluation of causative agents and their antifungal susceptibility patterns. TANAFFOS (Respiration) 20(1):29–35
Ghannoum MA, Rice LB (1999) Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 12(4):501–517
Coronavirus (COVID-19) hospitalizations - statistics and research [Internet]. Our World in Data. [cited 2021 Aug 15]. Available from: https://ourworldindata.org/covid-hospitalizations.
Acknowledgements
The authors acknowledge the members of the Community of Biotechnology, Dhaka, Bangladesh, for their support during the preparation of the manuscript.
Funding
The authors received no funding from external sources.
Author information
Authors and Affiliations
Contributions
YA conceived the study. NA, MM, MU, YA, and MH designed the study. NA and MM wrote the draft manuscript. MU, MH, NA, MM, AM, TR, and YA carried out the revisions. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmed, N., Mahmood, M.S., Ullah, M.A. et al. COVID-19-Associated Candidiasis: Possible Patho-Mechanism, Predisposing Factors, and Prevention Strategies. Curr Microbiol 79, 127 (2022). https://doi.org/10.1007/s00284-022-02824-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02824-6