Abstract
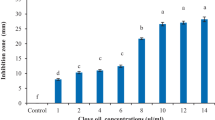
Dickeya zeae is a globally important bacterial pathogen that has been reported to cause severe soft rot diseases in several essential food crops, including bananas, rice, maize, and potatoes. Carvacrol, a hydrophobic terpene component, is found in aromatic plants of the Labiatae family and various essential oils. However, little work has been done on its antimicrobial potential against D. zeae. This study aimed to evaluate the antimicrobial activity and the functional mechanism of carvacrol against D. zeae. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of carvacrol against D. zeae were 0.1 mg/mL and 0.2 mg/mL, respectively. Carvacrol affected the cell membrane of D. zeae, as revealed by decreased intracellular ATP concentration, nucleic acid leakage, and decreased membrane potential. Scanning electron microscopy (SEM) micrographs confirmed that D. zeae cell membranes were damaged by carvacrol. Furthermore, a significant inhibition of D. zeae swimming motility and biofilm formation was observed following treatments with carvacrol at sub-inhibitory concentrations, indicating a significantly negative effect on these virulence factors. Accordingly, the tissue infection test revealed that carvacrol significantly reduced the pathogenicity of D. zeae. In a pot experiment, inoculated banana seedlings displayed remarkably lesser disease symptoms following treatment with carvacrol, and the control efficiency for banana soft rot was 32.0% at 14 days post-inoculation. To summarize, carvacrol exhibits strong antimicrobial activity against D. zeae and great potential applications in the control of D. zeae-associated crop diseases.




Similar content being viewed by others
References
Gardan L (2005) Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen. nov. as Dickeya chrysanthemi comb. nov. and Dickeya paradisiaca comb. nov. and delineation of four novel species, Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov., Dickeya dieffenbachiae sp. nov. and Dickeya zeae sp. nov. Int J Syst Evol Microbiol 55(1):1415–1427. https://doi.org/10.1099/ijs.0.02791-0
Hu M, Li J, Chen R, Li W, Feng L, Shi L, Xue Y, Feng X, Zhang L, Zhou J (2018) Dickeya zeae strains isolated from rice, banana and clivia rot plants show great virulence differentials. BMC Microbiol 18:136. https://doi.org/10.1186/s12866-018-1300-y
Li J, Hu M, Xue Y, Chen X, Lu G, Zhang L, Zhou J (2020) Screening, identification and efficacy evaluation of antagonistic bacteria for biocontrol of soft rot disease caused by Dickeya zeae. Microorganisms 8:697. https://doi.org/10.3390/microorganisms8050697
Lin B, Shen H, Pu X, Tian X, Zhao W, Zhu S, Dong M (2010) First report of a soft rot of banana in mainland China caused by a Dickeya sp. (Pectobacterium chrysanthemi). Plant Dis 94:640–640. https://doi.org/10.1094/PDIS-94-5-0640C
Zhang J, Shen H, Pu X, Lin B, Hu J (2014) Identification of Dickeya zeae as a causal agent of bacterial soft rot in banana in China. Plant Dis 98:436–442. https://doi.org/10.1094/PDIS-07-13-0711-RE
Czajkowski R, Perombelon M, Jafra S, Lojkowska E, Potrykus M, van der Wolf J, Sledz W (2015) Detection, identification and differentiation of Pectobacterium and Dickeya species causing potato blackleg and tuber soft rot: a review. Ann Appl Biol 166:18–38. https://doi.org/10.1111/aab.12166
Bogino PC, Oliva MDlM, Sorroche FG, Giordano W (2013) The role of bacterial biofilms and surface components in plant-bacterial associations. Int J Mol Sci 14:15838–15859. https://doi.org/10.3390/ijms140815838
Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13:614–629. https://doi.org/10.1111/j.1364-3703.2012.00804.x
Huang N, Pu X, Zhang J, Shen H, Yang Q, Wang Z, Lin B (2019) In vitro formation of Dickeya zeae MS1 biofilm. Curr Microbiol 76:100–107. https://doi.org/10.1007/s00284-018-1593-y
Wu J, Song Z, Xiang F, Zeng X, Gu Y (2009) Resistance mechanism of antagonistic bacterium in plant disease biocontrol. Hubei Agric Sci 9:2286–2288
Czajkowski R, Perombelon MC, van Veen JA, van der Wolf JM (2011) Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathol 60:999–1013. https://doi.org/10.1111/j.1365-3059.2011.02470.x
Antonia N, Teresa P (2012) Antimicrobial activity of carvacrol: current progress and future prospectives. Recent Pat Anti-Infect Drug Discov 7:28–35. https://doi.org/10.2174/157489112799829684
Gutierrez-Pacheco MM, Bernal-Mercado AT, Vázquez-Armenta FJ, González-Aguilar G, Lizardi-Mendoza J, Madera-Santana T, Nazzaro F, Ayala-Zavala J (2019) Quorum sensing interruption as a tool to control virulence of plant pathogenic bacteria. Physiol Mol Plant Pathol 106:281–291. https://doi.org/10.1016/j.pmpp.2019.04.002
Tapia-Rodriguez MR, Hernandez-Mendoza A, Gonzalez-Aguilar GA, Martinez-Tellez MA, Martins CM, Ayala-Zavala JF (2017) Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 75:255–261. https://doi.org/10.1016/j.foodcont.2016.12.014
Amini L, Soudi MR, Saboora A, Mobasheri H (2018) Effect of essential oil from Zataria multiflora on local strains of Xanthomonas campestris: an efficient antimicrobial agent for decontamination of seeds of Brassica oleracea var. capitata. Sci Hortic 236:256–264. https://doi.org/10.1016/j.scienta.2018.03.046
Nostro A, Cellini L, Zimbalatti V, Blanco AR, Marino A, Pizzimenti F, Giulio MD, Bisignano G (2012) Enhanced activity of carvacrol against biofilm of Staphylococcus aureus and Staphylococcus epidermidis in an acidic environment. APMIS 120:967–973. https://doi.org/10.1111/j.1600-0463.2012.02928.x
Sharifi-Rad M, Varoni EM, Iriti M, Martorell M, Setzer WN, del Mar CM, Salehi B, Soltani-Nejad A, Rajabi S, Tajbakhsh M (2018) Carvacrol and human health: a comprehensive review. Phytother Res 32:1675–1687. https://doi.org/10.1002/ptr.6103
Kang J, Liu L, Liu M, Wu X, Li J (2018) Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control 94:147–154. https://doi.org/10.1016/j.foodcont.2018.07.011
Sánchez E, García S, Heredia N (2010) Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl Environ Microbiol 76:6888–6894. https://doi.org/10.1128/AEM.03052-09
Shi C, Sun Y, Zheng Z, Zhang X, Song K, Jia Z, Chen Y, Yang M, Liu X, Dong R (2016) Antimicrobial activity of syringic acid against Cronobacter sakazakii and its effect on cell membrane. Food Chem 197:100–106. https://doi.org/10.1128/AEM.03052-09
Lan W, Zhang N, Liu S, Chen M, Xie J (2019) ε-Polylysine inhibits Shewanella putrefaciens with membrane disruption and cell damage. Molecules 24:3727. https://doi.org/10.3390/molecules24203727
Zhang Y, Liu X, Wang Y, Jiang P, Quek S (2016) Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 59:282–289. https://doi.org/10.1016/j.foodcont.2015.05.032
Shi C, Song K, Zhang X, Sun Y, Sui Y, Chen Y, Jia Z, Sun H, Sun Z, Xia X (2016) Antimicrobial activity and possible mechanism of action of citral against Cronobacter sakazakii. PLoS One 11:e0159006. https://doi.org/10.1371/journal.pone.0159006
Gutierrez-Pacheco M, Gonzalez-Aguilar G, Martinez-Tellez M, Lizardi-Mendoza J, Madera-Santana T, Bernal-Mercado A, Vazquez-Armenta F, Ayala-Zavala J (2018) Carvacrol inhibits biofilm formation and production of extracellular polymeric substances of Pectobacterium carotovorum subsp. carotovorum. Food Control 89:210–218. https://doi.org/10.1016/j.foodcont.2018.02.007
Stepanovic S (2000) A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179. https://doi.org/10.1016/S0167-7012(00)00122-6.26
Joshi JR, Burdman S, Lipsky A, Yedidia I (2015) Effects of plant antimicrobial phenolic compounds on virulence of the genus Pectobacterium. Res Microbiol 166:535–545. https://doi.org/10.1016/j.resmic.2015.04.004
Dixon RA (2001) Natural products and plant disease resistance. Nature 411:843–847. https://doi.org/10.1038/35081178
Cacciatore I, Di Giulio M, Fornasari E, Di Stefano A, Cerasa LS, Marinelli L, Turkez H, Di Campli E, Di Bartolomeo S, Robuffo I (2015) Carvacrol codrugs: a new approach in the antimicrobial plan. PLoS One 10:e0120937. https://doi.org/10.1371/journal.pone.0120937
Issam A-A, Zimmermann S, Reichling J, Wink M (2015) Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 22:245–255. https://doi.org/10.1016/j.phymed.2014.11.019
Shu H, Chen H, Wang X, Hu Y, Yun Y, Zhong Q, Chen W, Chen W (2019) Antimicrobial activity and proposed action mechanism of 3-Carene against Brochothrix thermosphacta and Pseudomonas fluorescens. Molecules 24:3246. https://doi.org/10.3390/molecules24183246
Ultee A, Kets EPW, Smid EJ (1999) Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 65:4606–4610. https://doi.org/10.1089/oli.1.1999.9.487
Burt SA (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022
Kaim G, Dimroth P (1999) ATP synthesis by F-type ATP synthase is obligatorily dependent on the transmembrane voltage. EMBO J 18:4118–4127. https://doi.org/10.1093/emboj/18.15.4118
Strahl H, Hamoen LW (2010) Membrane potential is important for bacterial cell division. Proc Natl Acad Sci USA 107:12281–12286. https://doi.org/10.1073/pnas.1005485107
Stratford JP, La Edwards C, Ghanshyam MJ, Malyshev D, Delise MA, Hayashi Y, Asally M (2019) Electrically induced bacterial membrane-potential dynamics correspond to cellular proliferation capacity. Proc Natl Acad Sci USA 116:9552–9557. https://doi.org/10.1101/542746
Liu X, Cai J, Chen H, Zhong Q, Hou Y, Chen W, Chen W (2020) Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb Pathog 141:103980. https://doi.org/10.1016/j.micpath.2020.103980
Kang J, Liu L, Wu X, Sun Y, Liu Z (2018) Effect of thyme essential oil against Bacillus cereus planktonic growth and biofilm formation. Appl Microbiol Biotechnol 102:10209–10218. https://doi.org/10.1007/s00253-018-9401-y
Shen S, Zhang T, Yuan Y, Lin S, Xu J, Ye H (2015) Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 47:196–202. https://doi.org/10.1016/j.foodcont.2014.07.003
Durand E, Lecomte J, Villeneuve P (2017) The biological and antimicrobial activities of phenolipids. Lipid Technol 29:67–70. https://doi.org/10.1002/lite.201700019
Chauhan AK, Kang SC (2014) Thymol disrupts the membrane integrity of Salmonella ser. typhimurium in vitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res Microbiol 165:559–565. https://doi.org/10.1016/j.resmic.2014.07.001
Inamuco J, Veenendaal AK, Burt SA, Post JA, Tjeerdsma-van Bokhoven JL, Haagsman HP, Veldhuizen EJ (2012) Sub-lethal levels of carvacrol reduce Salmonella Typhimurium motility and invasion of porcine epithelial cells. Vet Microbiol 157:200–207. https://doi.org/10.1016/j.vetmic.2011.12.021
Duan Q, Zhou M, Zhu L, Zhu G (2013) Flagella and bacterial pathogenicity. J Basic Microbiol 53:1–8. https://doi.org/10.1002/jobm.201100335
Jahn CE, Willis DK, Charkowski AO (2008) The flagellar sigma factor FliA is required for Dickeya dadantii virulence. Mol Plant Microbe Interact 21:1431–1442. https://doi.org/10.1094/MPMI-21-11-1431
Mina I, Jara N, Criollo J, Castillo J (2019) The critical role of biofilms in bacterial vascular plant pathogenesis. Plant Pathol 68:1439–1447. https://doi.org/10.1111/ppa.13073
Joshi JR, Khazanov N, Senderowitz H, Burdman S, Lipsky A, Yedidia I (2016) Plant phenolic volatiles inhibit quorum sensing in pectobacteria and reduce their virulence by potential binding to ExpI and ExpR proteins. Sci Rep 6:38126. https://doi.org/10.1038/srep38126
Burt SA, Ojo-Fakunle VT, Woertman J, Veldhuizen EJ (2014) The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS One 9:e93414. https://doi.org/10.1371/journal.pone.0093414
Acknowledgements
This research was supported by Grants from the Natural Science Foundation of Guangdong province (2015A030312002), the Science and Technology Project of Guangzhou city (201704030120), National Natural Science Foundation of China (31300118), Science and Technology Project of Guangdong province (2016B020202003), and the Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science (R2017PY-QY004, R2018QD-056).
Author information
Authors and Affiliations
Contributions
BL conceived and designed the experiments; SJ conducted the experiments and wrote the manuscript; JZ designed the experiments and revised the paper; ZW and QL designed the experiments; DS provided materials; QY, HS, and XP analyzed the data.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgements
This research was supported by Grants from the Natural Science Foundation of Guangdong province (2015A030312002), the Science and Technology Project of Guangzhou city (201704030120), National Natural Science Foundation of China (31300118), Science and Technology Project of Guangdong province (2016B020202003), and the Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science (R2017PY-QY004, R2018QD-056).
Rights and permissions
About this article
Cite this article
Jiang, S., Zhang, J., Yang, Q. et al. Antimicrobial Activity of Natural Plant Compound Carvacrol Against Soft Rot Disease Agent Dickeya zeae. Curr Microbiol 78, 3453–3463 (2021). https://doi.org/10.1007/s00284-021-02609-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02609-3