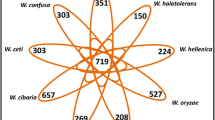
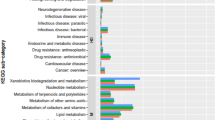
Abstract
Lactococcosis is a disease encountered in a wide variety of fish species causing mortalities and having great economic impact on farmed fish. In this study, we report for the first time the isolation of a strain of the recently described novel species Lactococcus petauri, from rainbow trout suffering from lactococcosis. The aim of this study was to determine the complete genome sequence of L. petauri strain LG_SAV_20 and to characterize its antimicrobial resistance and virulence. The genome of L. petauri LG_SAV_20 consists of 2,078,949 base pair (bp) with a GC content of 38.05%, 1950 predicted coding sequence (CDS), and 60 RNAs (51 tRNAs, 3 ncRNAs, and 6 rRNAs). Phylogenetic analysis revealed that L. petauri LG_SAV_20 shares most of its genome with L. garvieae strains isolated from rainbow trout. Detection of genes associated with antimicrobial resistance indicated that the isolate possesses the multidrug transporter mdt(A) gene, while using comparative analysis we identified several genes that might be related to bacterial pathogenesis. This genomic information provides new insights into the role of this novel species as an etiological agent of lactococcosis.




Similar content being viewed by others
References
Andrews, S (2010) FastQC-A quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/. Accessed 11 Mar 2019
Anthony J (1931) A note on capsule staining. Science 73:319–320
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA (2012) SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19(5):455–477
Bardou P, Mariette J, Escudie F, Djemiel C, Klopp C (2014) jvenn: an interactive Venn diagram viewer. BMC Bioinform 15:293. https://doi.org/10.1186/1471-2105-15-293
Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, Moller Aarestrup F, Hasman H (2014) In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58(7):3895–3903. https://doi.org/10.1128/AAC.02412-14
Chikhi R, Medvedev P (2014) Informed and automated k-mer size selection for genome assembly. Bioinformatics 30(1):31–37. https://doi.org/10.1093/bioinformatics/btt310
Clinical and Laboratory Standards Institute (CLSI) (2006) Methods for antimicrobial disk susceptibility testing of bacteria isolated from aquatic animals; approved guideline. CLSI Document VET03-A, Wayne
Dang HT, Park HK, Myung SC, Kim W (2012) Development of a novel PCR assay based on the 16S–23S rRNA internal transcribed spacer region for the detection of Lactococcus garvieae. J Fish Dis 35(7):481–487. https://doi.org/10.1111/j.1365-2761.2012.01382.x
Dean C, Noyes N, Lakin S, Rovira-Sanz P, Yang X, Belk K, Morley PS, Meinersmann R, Abdo Z (2018) Tychus: a whole genome sequencing pipeline for assembly, annotation and phylogenetics of bacterial genomes. bioRxiv. https://doi.org/10.1101/283101
Galperin MY, Makarova KS, Wolf YI, Koonin EV (2015) Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res 43:D261–269. https://doi.org/10.1093/nar/gku1223
Gibello A, Galan-Sanchez F, Blanco MM, Rodriguez-Iglesias M, Dominguez L, Fernandez-Garayzabal JF (2016) The zoonotic potential of Lactococcus garvieae: an overview on microbiology, epidemiology, virulence factors and relationship with its presence in foods. Res Vet Sci 109:59–70. https://doi.org/10.1016/j.rvsc.2016.09.010
Goodman LB, Lawton MR, Franklin-Guild RJ, Anderson RR, Schaan L, Thachil AJ, Wiedmann M, Miller CB, Alcaine SD, Kovac J (2017) Lactococcus petauri sp. nov., isolated from an abscess of a sugar glider. Int J Syst Evol Microbiol 67(11):4397–4404. https://doi.org/10.1099/ijsem.0.002303
Joensen KG, Scheutz F, Lund O, Hasman H, Kaas RS, Nielsen EM, Aarestrup FM (2014) Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J Clin Microbiol 52(5):1501–1510. https://doi.org/10.1128/JCM.03617-13
Kumar N, Lad G, Giuntini E, Kaye ME, Udomwong P, Shamsani NJ, Young JP, Bailly X (2015) Bacterial genospecies that are not ecologically coherent: population genomics of Rhizobium leguminosarum. Open Biol 5(1):140133. https://doi.org/10.1098/rsob.140133
Letunic I, Bork P (2016) Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44(W1):W242–245. https://doi.org/10.1093/nar/gkw290
Lin SH, Liao YC (2013) CISA: contig integrator for sequence assembly of bacterial genomes. PLoS ONE 8(3):e60843. https://doi.org/10.1371/journal.pone.0060843
Marcais G, Delcher AL, Phillippy AM, Coston R, Salzberg SL, Zimin A (2018) MUMmer4: a fast and versatile genome alignment system. PLoS Comput Biol 14(1):e1005944. https://doi.org/10.1371/journal.pcbi.1005944
Morita H, Toh H, Oshima K, Yoshizaki M, Kawanishi M, Nakaya K, Suzuki T, Miyauchi E, Ishii Y, Tanabe S, Murakami M, Hattori M (2011) Complete genome sequence and comparative analysis of the fish pathogen Lactococcus garvieae. PLoS ONE 6(8):e23184. https://doi.org/10.1371/journal.pone.0023184
Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, Fookes M, Falush D, Keane JA, Parkhill J (2015) Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31(22):3691–3693. https://doi.org/10.1093/bioinformatics/btv421
Palacios A, Zamora J, Velazquez J, Zamora E, Duran A (1993) Streptococcosis in rainbow trout (Oncorhynchus mykiss) in Spain. Bollettino Societa Italiana di Patologia Ittica 5:11–16
Peng Y, Leung M, Yiu M, Chin L (2010) IDBA—a practical iterative de Bruijn Graph De Novo Assembler. In: Berger B (ed) Research in computational molecular biology. Lecture notes in computer science, vol 6044. Springer, Berlin
Perreten V, Schwarz FV, Teuber M, Levy SB (2001) Mdt(A), a new efflux protein conferring multiple antibiotic resistance in Lactococcus lactis and Escherichia coli. Antimicrob Agents Chemother 45(4):1109–1114. https://doi.org/10.1128/AAC.45.4.1109-1114.2001
Price MN, Dehal PS, Arkin AP (2010) FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE 5(3):e9490. https://doi.org/10.1371/journal.pone.0009490
Richter M, Rossello-Mora R (2009) Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA 106(45):19126–19131. https://doi.org/10.1073/pnas.0906412106
Savvidis K, Anatoliotis C, Kanaki Z, Vafeas G (2007) Epizootic outbreaks of Lactococcosis disease in rainbow trout, Oncorhynchus mykiss (Walbaum), culture in Greece. Bull Eur Assoc Fish Pathol 27:223–228
Schmidtke LM, Carson J (2003) Antigen recognition by rainbow trout (Oncorhynchus mykiss) of whole cell proteins expressed by Lactococcus garvieae when obtained directly from fish and under iron limited culture conditions. Vet Microbiol 93(1):63–71. https://doi.org/10.1016/S0378-1135(02)00440-6
Seemann T (2014) Prokka: rapid prokaryotic genome annotation. Bioinformatics 30(14):2068–2069. https://doi.org/10.1093/bioinformatics/btu153
Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJ, Birol I (2009) ABySS: a parallel assembler for short read sequence data. Genome Res 19(6):1117–1123. https://doi.org/10.1101/gr.089532.108
Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J (2016) NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 44(14):6614–6624. https://doi.org/10.1093/nar/gkw569
Walther C, Rossano A, Thomann A, Perreten V (2008) Antibiotic resistance in Lactococcus species from bovine milk: presence of a mutated multidrug transporter mdt(A) gene in susceptible Lactococcus garvieae strains. Vet Microbiol 131(3–4):348–357. https://doi.org/10.1016/j.vetmic.2008.03.008
Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV (2012) Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67(11):2640–2644. https://doi.org/10.1093/jac/dks261
Zerbino DR, Birney E (2008) Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 18(5):821–829. https://doi.org/10.1101/gr.074492.107
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All the animal procedures were performed in compliance with the Greek legislation and the protocol was approved by the Ethical Committee of the Veterinary Research Institute, ELGO-Demeter.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kotzamanidis, C., Malousi, A., Bitchava, K. et al. First Report of Isolation and Genome Sequence of L. petauri Strain from a Rainbow Trout Lactococcosis Outbreak. Curr Microbiol 77, 1089–1096 (2020). https://doi.org/10.1007/s00284-020-01905-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-01905-8