Abstract
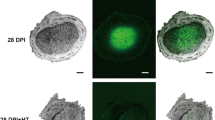
Legumes interact with symbiotic rhizobia to produce nitrogen-fixation root nodules under nitrogen-limiting conditions. The contribution of glutathione (GSH) to this symbiosis and anti-oxidative damage was investigated using the M. huakuii gshB (encoding GSH synthetase) mutant. The gshB mutant grew poorly with different monosaccharides, including glucose, sucrose, fructose, maltose, or mannitol, as sole sources of carbon. The antioxidative capacity of gshB mutant was significantly decreased by these treatments with H2O2 under the lower concentrations and cumene hydroperoxide (CUOOH) under the higher concentrations, indicating that GSH plays different roles in response to organic peroxide and inorganic peroxide. The gshB mutant strain displayed no difference in catalase activity, but significantly lower levels of the peroxidase activity and the glutathione reductase activity than the wild type. The same level of catalase activity could be associated with upregulation of the transcriptional activity of the catalase genes under H2O2-induced conditions. The nodules infected by the gshB mutant were severely impaired in abnormal nodules, and showed a nodulation phenotype coupled to a 60% reduction in the nitrogen fixation capacity. A 20-fold decrease in the expression of two nitrogenase genes, nifH and nifD, is observed in the nodules induced by gshB mutant strain. The symbiotic deficiencies were linked to bacteroid early senescence.




Similar content being viewed by others
References
Collinson EJ, Wheeler GL, Garrido EO, Avery AM, Avery SV, Grant CM (2002) The yeast glutaredoxins are active as glutathione peroxidases. J Biol Chem 277(19):16712–16717
Imlay JA (2003) Pathway of oxidative damage. Annu Rev Microbiol 57:395–418
Liu L, Xu P, Zeng G, Huang D, Zhao M, Lai C, Chen M, Li N, Huang C, Wang C, Cheng M, He X, Lai M, He Y (2014) Inherent antioxidant activity and high yield production of antioxidants in Phanerochaete chrysosporium. Biochem Eng J 90:245–254
Bräutigam L, Zhang J, Dreij K, Spahiu L, Holmgren A, Abe H, Tew KD, Townsend DM, Kelner MJ, Morgenstern R, Johansson K (2018) A GSH transferase/peroxidase essential for development and hematopoietic stem cell differentiation. Redox Biol 17:171–179
Grant CM, Collinson L, Roe JH, Dawes IW (2010) Yeast glutathione reductase is required for protection against oxidative stress and is a target gene for yap-1 transcriptional regulation. Mol Microbiol 21(1):171–179
Vergauwen B, Pauwels F, Jacquemotte F, Meyer TE, Cusanovich MA, Bartsch RG, Van Beeumen JJ (2001) Characterization of glutathione amide reductase from chromatium gracile identification of a novel thiol peroxidase (prx/grx) fueled by glutathione amide redox cycling. J Biol Chem 276(24):20890–20897
Li M, Zhang Z, Yuan J, Zhang Y, Jin X (2014) Altered glutamate cysteine ligase expression and activity in renal cell carcinoma. Biomed Rep 2:831–834
Xu Y, Murooka Y (1995) A large plasmid isolated from Rhizobium huakuii bv. renge that includes genes for both nodulation of Astragalus sinicus cv. japan and nitrogen fixation. J Ferment Bioeng 80(3):276–279
Cheng G, Li Y, Xie B, Yang C, Zhou J (2007) Cloning and identification of lpsH, a novel gene playing a fundamental role in symbiotic nitrogen fixation of Mesorhizobium huakuii. Curr Microbiol 54(5):371–375
Bladergroen MR, Spaink HP (1998) Genes and signal molecules involved in the Rhizobia-leguminoseae symbiosis. Curr Opin Plant Biol 1(4):353–359
Guan D, Stacey N, Liu C, Wen J, Murray JD (2013) Rhizobial infection is associated with the development of peripheral vasculature in nodules of Medicago truncatula. Plant physiol 162(1):107–115
Yang C, Signer ER, Hirsch AM (1992) Nodules initiated by Rhizobium meliloti exopolysaccharide mutants lack a discrete, persistent nodule meristem. Plant Physiol 98(1):143–151
Wang Q, Yang S, Liu J, Terecskei K, Ábrahám E, Gombár A, Domonkosc A, Szücs A, Körmöczib P, Wang T, Fodorc L, Maod L, Feid Z, Kondorosib E, Kalóc P, Keresztb A, Zhua H (2017) Host-secreted antimicrobial peptide enforces symbiotic selectivity in Medicago truncatula. Proc Natl Acad Sci USA 114(26):6854–6859
Damiani I, Pauly N, Puppo A, Brouquisse R, Boscari A (2016) Reactive oxygen species and nitric oxide control early steps of the legume—Rhizobium symbiotic interaction. Front Plant Sci 7:454
Harrison J, Jamet A, Muglia CI, Van de G, Aguilar OM, Puppo A, Frendo P (2005) Glutathione plays a fundamental role in growth and symbiotic capacity of Sinorhizobium meliloti. J Bacteriol 187(1):168–174
Ra Taté, Cermola M, Riccio A, Diez-Roux G, Patriarca EJ (2012) Glutathione is required by Rhizobium etli for glutamine utilization and symbiotic effectiveness. Mol Plant Microbe In 25(3):331–340
Beringer JE, Hopwood DA (1976) Chromosomal recombination and mapping in Rhizobium leguminosarum. Nature 264:291–293
Poole PS, Blyth A, Reid C, Walters K (1994) myo-Inositol catabolism and catabolite regulation in Rhizobium leguminosarum bv viciae. Microbiology 140:2787–2795
Karunakaran R, Haag AF, East AK, Ramachandran VK, Prell J, James EK, Scocchi M, Ferguson GP, Poole PS (2010) BacA is essential for bacteroid development in nodules of galegoid, but not phaseoloid, legumes. J Bacteriol 192:2920–2928
Karunakaran R, Ebert K, Harvey S, Leonard ME, Ramachandran V, Poole PS (2006) Thiamine is synthesized by a salvage pathway in Rhizobium leguminosarum bv. viciae strain 3841. J Bacteriol 188:6661–6668
Hider RC, Kong XL (2011) Glutathione: a key component of the cytoplasmic labile iron pool. Biometals 24:1179–1187
Allaway B, Lodwig E, Crompton LA, Wood M, Parsons R, Wheeler TR, Poole PS (2000) Identification of alanine dehydrogenase and its role in mixed secretion of ammonium and alanine by pea bacteroids. Mol Microbiol 36:508–515
Yan H, Xiao G, Zhang J, Hu Y, Yuan F, Cole DK, Zheng C, Gao GF (2004) SARS coronavirus induces apoptosis in Vero E6 cells. J Med Virol 73:323–331
Mulley G, White JP, Karunakaran R, Prell J, Bourdes A, Bunnewell S, Hill L, Poole PS (2011) Mutation of gogat prevents pea bacteroid formation and N2 fixation by globally downregulating transport of organic nitrogen sources. Mol Microbiol 80(1):149–167
Prell J, Bourdès A, Karunakaran R, Lopez-Gomez M, Poole PS (2009) Pathway of {gamma}-aminobutyrate (GABA) metabolism in Rhizobium leguminosarum 3841 and its role in symbiosis. J Bacteriol 191:2177–2186
Alam K, Ghousunnissa S, Nair S, Valluri VL, Mukhopadhyay S (2010) Glutathione-redox balance regulates c-rel-driven il-12 production in macrophages: possible implications in antituberculosis immunotherapy. J Immunol 184(6):2918–2929
Chen KG, Szakács G, Annereau JP, Rouzaud F, Liang XJ, Valencia JC, Nagineni CN, Hooks JJ, Hearing VJ, Gottesman MM (2010) Principal expression of two mRNA isoforms (ABCB5α and ABCB5β) of the ATP-binding cassette transporter gene ABCB5 in melanoma cells and melanocytes. Pigment Cell Res 18(2):102–112
Wei Q, Marc L, Carpenter EP, Shirihai OS, Van VHW (2015) ATP binding and hydrolysis properties of ABCB10 and their regulation by glutathione. PLoS ONE 10(6):e0129772
Todd JD, Wexler M, Sawers G, Yeoman KH, Poole PS, Johnston AW (2002) RirA, an iron-responsive regulator in the symbiotic bacterium Rhizobium leguminosarum. Microbiology 148(12):4059–4071
Chao TC, Buhrmester J, Hansmeier N, Puhler A, Weidner S (2005) Role of the regulatory gene rirA in the transcriptional response of Sinorhizobium meliloti to iron limitation. Appl Environ Microb 71(10):5969–5982
Benyamina SM, Baldacci-Cresp F, Couturier J, Chibani K, Hopkins J, Bekki A, de Lajudie P, Rouhier N, Jacquot JP, Alloing G, Puppo A, Frendo P (2013) Two Sinorhizobium meliloti glutaredoxins regulate iron metabolism and symbiotic bacteroid differentiation. Environ Microbiol 15:795–810
Tal S, Chun TW, Gavini N, Burgess BK (1991) The delta nifB (or delta nifE) femo cofactor-deficient mofe protein is different from the delta nifh protein. J Biol Chem 266(16):10654–10657
Greenberg JT, Demple B (1986) Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J Bacteriol 168:1026–1029
Grant CM, MacIver FH, Dawes IW (1997) Glutathione synthetase is dispensable for growth under both normal and oxidative stress conditions in the yeast Saccharomyces cerevisiae due to the accumulation of the dipeptide gamma-glutamylcysteine. Mol Biol Cell 8:1699–1707
Prell J, Whit JP, Bourdes A, Bunnewell S, Bongaerts RJ, Poole PS (2009) Legumes regulate rhizobium bacteroid development and persistence by the supply of branched-chain amino acids. Proc Natl Acad Sci USA 106(30):12477–12482
Engelmann J, Volk J, Leyhausen G, Geurtsen W (2010) Ros formation and glutathione levels in human oral fibroblasts exposed to tegdma and camphorquinone. J Biomed Mater Res B Appl Biomater 75(2):272–276
Rahantaniaina MS, Li S, Chatel-Innocenti G, Tuzet A, Mhamdi A, Vanacker H, Noctor G (2017) Glutathione oxidation in response to intracellular H2O2: key but overlapping roles for dehydroascorbate reductases. Plant Signal Behav 12(8):e1356531
Song J, Kang SM, Lee WT, Park KA, Lee KM, Lee JE (2014) Glutathione protects brain endothelial cells from hydrogen peroxide-induced oxidative stress by increasing nrf2 expression. Exp Neurobiol 23(1):93–103
Sedighi O, Zargari M, Varshi G (2014) Effect of selenium supplementation on glutathione peroxidase enzyme activity in patients with chronic kidney disease: a randomized clinical trial. Nephro Urol Mon 6(3):e17945
Vergauwen B, Pauwels F, Vaneechoutte M, Beeumen JJV (2003) Exogenous glutathione completes the defense against oxidative stress in Haemophilus influenzae. J Bacteriol 185(5):1572–1581
Giergiel M, Lopucki M, Stachowicz N, Kankofer M (2012) The influence of age and gender on antioxidant enzyme activities in humans and laboratory animals. Aging Clin Exp Res 24(6):561–569
Cheng G, Karunakaran R, East AK, Munozazcarate O, Poole PS (2017) Glutathione affects the transport activity of Rhizobium leguminosarum 3841 and is essential for efficient nodulation. Fems Microbiol Lett 364(8):fnx045
Xi J, Chen Y, Nakashima J, Wang S, Chen R (2013) Medicago truncatula esn1 defines a genetic locus involved in nodule senescence and symbiotic nitrogen fixation. Mol Plant Microbe In 26(8):893–902
Kovach ME, Phillips RW, Elzer PH, Roop RMI, Peterson KM (1994) pBBR1MCS: a broad-host-range cloning vector. Biotechniques 16(5):800–802
Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145(1):69–73
Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid rk2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76(4):1648–1652
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST(c)) for group-wise comparison and statistical analysis of relative expression results in Real-time PCR. Nucleic Acids Res 30:E36
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31772399) and the Fundamental Research Funds for the Central Universities, South-Central University for Nationalities (Grant No. CZY18022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, S., Yin, J., Peng, Y. et al. Glutathione is Involved in Detoxification of Peroxide and Root Nodule Symbiosis of Mesorhizobium huakuii. Curr Microbiol 77, 1–10 (2020). https://doi.org/10.1007/s00284-019-01784-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-019-01784-8