Abstract
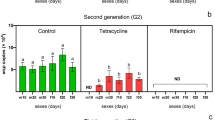
Wolbachia are the most common symbionts in arthropods; antibiotic treatment for eliminating the symbionts from their host is necessary to investigate the functions. Tetracycline antibiotics are widely used to remove endosymbiont Wolbachia from insect hosts. However, very little has been known on the effects of tetracycline on population size of Wolbachia in small brown planthopper (SBPH), Laodelphax striatellus (Fallén), an important insect pest of rice in Asia. Here, we investigated the dynamics of Wolbachia population density in females and males of L. striatellus by real-time fluorescent quantitative PCR method. The Wolbachia density in females and males of L. striatellus all declined sharply after treatment with 2 mg/mL tetracycline for one generation, and continued to decrease to a level which could not be detected by both qPCR and diagnostic PCR after treated for another generation, then maintained at 0 in the following three generations with continuous antibiotic treatment. Wolbachia infection did not recover in L. striatellus after stopping tetracycline treatment for ten generations. This is the first report to precisely monitor the population dynamics of Wolbachia in L. striatellus during successive tetracycline treatment and after that. The results provide a useful method for evaluating the efficiency of artificial operation of endosymbionts.


Similar content being viewed by others
References
Jeyaprakash A, Hoy MA (2000) Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty—three arthropod species. Insect Mol Biol 9:393–405
Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH (2008) How many species are infected with Wolbachia?—a statistical analysis of current data. FEMS Microbiol Lett 281:215–220
Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ (2015) The incidence of bacterial endosymbionts in terrestrial arthropods. Proc R Soc B 282:20150249
Teixeira L, Ferreira Á, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Bio 6:e1000002
Hedges LM, Brownlie JC, O'Neill SL, Johnson KN (2008) Wolbachia and virus protection in insects. Science 322:702
Vasanthakrishnan RB, Vanika G, Siva-Jothy JA, Monteith KM, Brown SP, Vale PF (2016) Wolbachia confers sex-specific resistance and tolerance to enteric but not systemic bacterial infection in Drosophila. BioRxiv. https://doi.org/10.1101/045757
Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Hedges M, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, Hurk AF, Ryan PA, O'Neill SL (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139:1268–1278
Bian GW, Xu Y, Lu P, Xie Y, Xi ZY (2010) The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6:e1000833
Bian GW, Joshi D, Dong YM, Lu P, Zhou GL, Pan XL, Xu Y, Dimopoulos G, Xi ZY (2013) Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340:748–751
Berticat C, Rousset F, Raymond M, Berthomieu A, Weill M (2002) High Wolbachia density in insecticide–resistant mosquitoes. Proc Roy Soc B 269:1413–1416
Duron O, Labbé P, Berticat C, Rousset F, Guillot S, Raymond M, Weill M (2006) High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution 60:303–314
Hoerauf A, Nissen-Pähle K, Schmetz C, Henkle-Dührsen K, Blaxter ML, Büttner DW, Gallin MY, Al-Qaoud KM, Lucius R, Fleischer B (1999) Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J Clin Invest 103:11–18
Hoerauf A, Volkmann L, Nissen-Paehle K, Schmetz C, Autenrieth I, Büttner DW, Fleischer B (2000) Targeting of Wolbachia endobacteria in Litomosoides sigmodontis: comparison of tetracyclines with chloramphenicol, macrolides and ciprofloxacin. Trop Med Int Health 5:275–279
Wright JD, Wang BT (1980) Observations on Wolbachiae in mosquitoes. J Invertebr Pathol 35:200–208
Trpis M, Perrone JB, Reissig M, Parker KL (1981) Control of cytoplasmic incompatibility in the Aedes scutellaris complex incompatible crosses become compatible by treatment of larvae with heat or antibiotics. J Hered 72:313–317
Graham RI, Grzywacz D, Mushobozi WL, Wilson K (2012) Wolbachia in a major African crop pest increases susceptibility to viral disease rather than protects. Ecol Lett 15:993–1000
Sanada-Morimura S, Matsumura M, Noda H (2013) Male Killing caused by a Spiroplasma symbiont in the small brown planthopper, Laodelphax striatellus. J Hered 104:821–829
Wolfgang A, Markus R, Dimitrios N, Christian S (2009) Evidence for low-titre infections in insect symbiosis: Wolbachia in the bark beetle Pityogenes chalcographus (Coleoptera, Scolytinae). Environ Microbiol 11:1923–1933
Schneider DI, Garschall KI, Parker AG, Abd-Alla AM, Miller WJ (2013) Global Wolbachia prevalence, titer fluctuations and their potential of causing cytoplasmic incompatibilities in tsetse flies and hybrids of Glossina morsitans subgroup species. J Invertebr Pathol 112:S104–S115
Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P, Lloyd AL, Ritchie SA, O’Neill SL, Hoffmann AA (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476:450
Serbus LR, White PM, Silva JP, Rabe A, Teixeira L, Albertson R, Sullivan W (2015) The impact of host diet on Wolbachia titer in Drosophila. PLoS Pathog 11:e1004777
Monnin D, Kremer N, Berny C, Henri H, Dumet A, Voituron Y, Desouhant E, Vavre F (2016) Influence of oxidative homeostasis on bacterial density and cost of infection in Drosophila-Wolbachia symbioses. J Evol Biol 29:1211–1222
Nault LR (1994) Transmission biology, vector specificity and evolution of planthopper-transmitted plant viruses. In: Nault LR (ed) Planthoppers. Springer, Boston, pp 429–448
Otuka A, Matsumura M, Sanada-Morimura S, Takeuchi H, Watanabe T, Ohtsu R, Inoue H (2010) The 2008 overseas mass migration of the small brown planthopper, Laodelphax striatellus, and subsequent outbreak of rice stripe disease in western Japan. Appl Entomol Zool 45:259–266
Gao B, Wu J, Huang S, Mu L, Han Z (2007) Insecticide resistance in field populations of Laodelphax striatellus Fallén (Homoptera: Delphacidae) in China and its possible mechanisms. Int J Pest Manag 54:13–19
Li Y, Liu X, Guo H (2018) Variations in endosymbiont infection between buprofezin-resistant and susceptible strains of Laodelphax striatellus (Fallén). Curr Microbiol 75:709–715
Hoshizaki S, Shimada T (1995) PCR-based detection of Wolbachia, cytoplasmic incompatibility microorganisms, infected in natural populations of Laodelphax striatellus (homoptera: delphacidae) in central japan: has the distribution of Wolbachia spread recently? Insect Mol Biol 4:237–243
Noda H, Koizumi Y, Zhang Q, Deng KJ (2001) Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insect Biochem Molec 31:727–737
Zhang YL, Guo HF, Yang Q, Li S, Wang LH, Zhang GF, Fang JC (2012) Overexpression of a P450 gene (CYP6CW1) in buprofezin-resistant Laodelphax striatellus (Fallén). Pestic Biochem Phys 104:277–282
Koch C, Rainey FA, Stackebrandt E (1994) 16S rDNA studies on members of Arthrobacter and Micrococcus: an aid for their future taxonomic restructing. FEMS Microbiol Lett 123:167–171
Zhou WG, Rousset F, O'Neill S (1998) Phylogeny and PCR–based classification of Wolbachia strains using wsp gene sequences. Proc R Soc B 265:509–515
Thao ML, Baumann P (2004) Evidence for multiple acquisition of Arsenophonus by whitefly species (Sternorrhyncha: Aleyrodidae). Curr Microbiol 48:140–144
Vanbroekhoven K, Ryngaert A, Wattiau P, Mot R, Springael D (2004) Acinetobacter diversity in environmental samples assessed by 16S rRNA gene PCR–DGGE fingerprinting. FEMS Microbiol Ecol 50:37–50
Alonso C, Warnecke F, Amann R, Pernthaler J (2007) High local and global diversity of Flavobacteria in marine plankton. Environ Microbiol 9:1253–1266
Zhu H, Sun SJ, Dang HY (2008) PCR detection of Serratia spp. using primers targeting pfs and luxS genes involved in AI-2-dependent quorum sensing. Curr Microbiol 57:326–330
Nakamura Y, Kawai S, Yukuhiro F, Ito S, Gotoh T, Kisimoto R, Yanase T, Matsumoto Y, Kageyama D, Noda H (2009) Prevalence of Cardinium bacteria in planthoppers and spider mites and taxonomic revision of “Candidatus Cardinium hertigii” based on detection of a new Cardinium group from biting midges. Appl Environ Microb 75:6757–6763
Zhou LL, Zhang KJ, Song ZW, Hong XX (2010) Relationship of WO phage and Wolbachia infection in Laodelphax striatellus (Fallén) (Hemiptera: Delphacidae). Acta Entomol Sin 53:978–984
Dossi FCA, Silva EP, Cônsoli FL (2014) Population dynamics and growth rates of endosymbionts during Diaphorina citri (Hemiptera, Liviidae) ontogeny. Microb Ecol 68:881–889
Whelan JA, Russell NB, Whelan MA (2003) A method for the absolute quantification of cDNA using real-time PCR. J Immunol Methods 278:261–269
Bustin SA, Benes V, Nolan T, Pfaffl MW (2005) Quantitative real-time RT-PCR—a perspective. J Mol Endocrinol 34:597–601
Kubista M, Andrade JM, Bengtsson M, Forootan A, Jonák J, Linda K, Sindelka R, Sjöback R, Sjögreen B, Strömbom L, Ståhlberg A, Zoric N (2006) The real-time polymerase chain reaction. Mol Aspects Med 27:95–125
VanGuilder HD, Vrana KE, Freeman WM (2008) Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques 44:619–626
Garrido-Maestu A, Chapela MJ, Vieites JM, Cabado AG (2015) lolB gene, a valid alternative for qPCR detection of Vibrio cholerae in food and environmental samples. Food Microbiol 46:535–540
Steeples LR, Guiver M, Jones NP (2016) Real-time PCR using the 529 bp repeat element for the diagnosis of atypical ocular toxoplasmosis. Brit J Ophthalmol 100:200–203
Li YY, Floate KD, Fields PG, Pang BP (2014) Review of treatment methods to remove Wolbachia bacteria from arthropods. Symbiosis 62:1–15
Zhong Y, Li ZX (2013) Influences of tetracycline on the reproduction of the B biotype of Bemisia tabaci (Homoptera: Aleyrodidae). Appl Entomol Zool 48:241–246
Zha XF, Zhang WJ, Zhou CY, Zhang LY, Xiang ZH, Xia QY (2014) Detection and characterization of Wolbachia infection in silkworm. Genet Mol Biol 37:573–580
Pike N, Kingcombe R (2009) Antibiotic treatment leads to the elimination of Wolbachia endosymbionts and sterility in the diplodiploid collembolan Folsomia candida. BMC Biol 7:54
Grenier S, Gomes SM, Pintureau B, Lassablière F, Bolland P (2002) Use of tetracycline in larval diet to study the effect of Wolbachia on host fecundity and clarify taxonomic status of Trichogramma species in cured bisexual lines. J Invertebr Pathol 80:13–21
Acknowledgements
We thank Yueliang Zhang at the Institute of Plant Protection, Jiangsu Academy of Agricultural Sciences, China, for collecting the small brown planthopper, Laodelphax striatellus.
Funding
This research was supported by grants from the National Natural Science Foundation of China (Grant No. 31672027) and the Independent Innovation Fund of Agricultural Science and Technology in Jiangsu province, China (cx(16)1001).
Author information
Authors and Affiliations
Contributions
HG conceived the experiments. HG designed the experiments. YL collected the data. HG, YL, and XL analyzed the data. YL and HG wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Liu, X. & Guo, H. Population Dynamics of Wolbachia in Laodelphax striatellus (Fallén) Under Successive Stress of Antibiotics. Curr Microbiol 76, 1306–1312 (2019). https://doi.org/10.1007/s00284-019-01762-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-019-01762-0