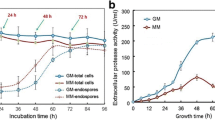
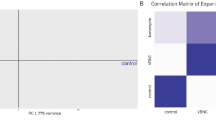
Abstract
Biological science discovery often involves comparing conditions to a normal state, but little is known about “normal.” Therefore, we used proteomic strategy to compare data from replicate samples of Bacillus subtilis 168 which were grown under identical condition. The results show that 294 differentially expressed proteins were annotated in 88 Gene Ontology functional groups and enriched in 13 KEGG pathways. We assume that normal expression differences are associated with adaptation to diverse environments. Moreover, five proteins (CotY, ThiG, SspA, SspB, and SspE) and their related genes were identified as having significantly different expressions at translational and transcriptional levels. Most of them are related to stress resistance and germination, indicating that normal expression differences can be regarded as a rapid response mechanism for survival. However, unstable protein expression may cause some fermentative problems that were observed in histidine and sulfur metabolism pathways. Our study facilitates dissection of the influence of biological variance on cultivation safety and stability.





Similar content being viewed by others
References
Budde I, Steil L, Scharf C, Völker U, Bremer E (2006) Adaptation of Bacillus subtilis to growth at low temperature: a combined transcriptomic and proteomic appraisal. Microbiology 152(Pt 3):831–853
Caplice E, Fitzgerald GF (1999) Food fermentations: role of microorganisms in food production and preservation. Int J Food Microbiol 50(1–2):131–149
Charles M (1985) Fermentation scale-up: problems and possibilities. Trends Biotechnol 3(6):134–139
Chen RX, Song HY, Dong YY, Hu C, Zheng QD, Xue TC, Liu XH, Zhang Y, Chen J, Ren ZG (2014) Dynamic expression patterns of differential proteins during early invasion of hepatocellular carcinoma. PLoS ONE 9(3):e88543
Diaz-Ricci JC, Regan L, Bailey JE (1991) Effect of alteration of the acetic acid synthesis pathway on the fermentation pattern of escherichia coli. Biotechnol Bioeng 38(11):1318–1324
Du C, Liang JR, Chen DD, Xu B, Zhuo WH, Gao YH, Chen CP, Bowler C, Zhang W (2014) iTRAQ-based proteomic analysis of the metabolism mechanism associated with silicon response in the marine diatom Thalassiosira pseudonana. J Proteome Res 13(2):720–734
Hajo Z, Christoph E, Lars W, Bernd B, Ralf R (2011) Biological versus technical variability in 2-D DIGE experiments with environmental bacteria. Proteomics 11(16):3380–3389
Hao Z, Yan L, Liu J, Song F, Zhang J, Li X (2015) Extraction of antibiotic zwittermicin A from Bacillus thuringiensis by macroporous resin and silica gel column chromatography. Biotechnol Appl Biochem 62(3):369–374. https://doi.org/10.1002/bab.1277
Helmann JD, Wu MFW, Kobel PA, Gamo FJ, Wilson M, Morshedi MM, Navre M, Paddon C (2001) Global transcriptional response of Bacillus subtilis to heat shock. J Bacteriol 183(24):7318–7328
Higdon R, Kolker E (2015) Can “normal” protein expression ranges be estimated with high-throughput proteomics? J Proteome Res 14(6):2398–2407. https://doi.org/10.1021/acs.jproteome.5b00176
Johnson MJ, Todd SJ, Ball DA, Shepherd AM, Sylvestre P, Moir A (2006) ExsY and CotY are required for the correct assembly of the exosporium and spore coat of Bacillus cereus. J Bacteriol 188(22):7905–7913
Kamran A, Bibi Z, Aman A, Qader SAU (2016) Lactose hydrolysis approach: Isolation and production of β-galactosidase from newly isolated Bacillus strain B-2. Biocatal Agric Biotechnol 5:99–103. https://doi.org/10.1016/j.bcab.2015.12.010
Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessières P, Bolotin A, Borchert S (1997) The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390(6657):249–256
Maaβ S, Wachlin G, Bernhardt J, Eymann C, Fromion V, Riedel K, Becher D, Hecker M (2014) Highly precise quantification of protein molecules per cell during stress and starvation responses in Bacillus subtilis. Mol Cell Proteomics 13(9):2260–2276
Malherbe S, Bauer FF, Toit MD (2007) Understanding problem fermentations: a review. S Afr J Enol Viticult 28(2):169–186
Parekh S, Vinci VA, Strobel RJ (2000) Improvement of microbial strains and fermentation processes. Appl Microbiol Biotechnol 54(3):287–301
Park JH, Dorrestein PC, Zhai H, Kinsland C, And ML, Begley TP (2003) Biosynthesis of the thiazole moiety of thiamin pyrophosphate (Vitamin B1)†. Biochemistry 42(42):12430–12438
Petersohn A, Brigulla M, Haas S, Hoheisel JD, Völker U, Hecker M (2001) Global analysis of the general stress response of Bacillus subtilis. J Bacteriol 183(19):5617–5631
Ploss TN, Reilman E, Monteferrante CG, Denham EL, Piersma S, Lingner A, Vehmaanpera J, Lorenz P, van Dijl JM (2016) Homogeneity and heterogeneity in amylase production by Bacillus subtilis under different growth conditions. Microb Cell Fact 15:57. https://doi.org/10.1186/s12934-016-0455-1
Price CW, Fawcett P, Su N, Murphy CK, Youngman P (2001) Genome-wide analysis of the general stress response in Bacillus subtilis. Mol Microbiol 41(4):757–774
Reder A, Höper D, Weinberg C, Gerth U, Fraunholz M, Hecker M (2008) The Spx paralogue MgsR (YqgZ) controls a subregulon within the general stress response of Bacillus subtilis. Mol Microbiol 69(5):1104–1120
Reis MAM, Almeida JS, Lemos PC, Carrondo MJT (1992) Effect of hydrogen sulfide on growth of sulfate reducing bacteria. Biotechnol Bioeng 40(5):593–600
Sanchez-Salas JL, Santiago-Lara ML, Setlow B, Sussman MD, Setlow P (1992) Properties of Bacillus megaterium and Bacillus subtilis mutants which lack the protease that degrades small, acid-soluble proteins during spore germination. J Bacteriol 174(3):807–814
Schallmey M, Singh A, Ward OP (2004) Developments in the use of Bacillus species for industrial production. Can J Microbiol 50(1):1–17. https://doi.org/10.1139/w03-076
Schwechheimer SK, Park EY, Revuelta JL, Becker J, Wittmann C (2016) Biotechnology of riboflavin. Appl Microbiol Biotechnol 100(5):2107–2119. https://doi.org/10.1007/s00253-015-7256-z
Srianta I, Nugerahani I, Sutedja AM, Widharna RM (2014) Optimization of drying temperature and water extraction time of Monascus-fermented durian seed for the Monacolin K content using response surface methodology. Int Food Res J 21(1):73–75
Sun H, Liu X, Li F, Wei L, Jing Z, Xiao Z, Shen L, Ying L, Wang F, Yang J (2017) First comprehensive proteome analysis of lysine crotonylation in seedling leaves of Nicotiana tabacum. Sci Rep 7(1):3013
Sun X, Yang P, Xuan LI, Wang Y (2010) Study Progress of fermentation conditions of L-histidine and screening of high productive strain. China Brewing
Valcu CM, Valcu M (2007) Reproducibility of two-dimensional gel electrophoresis at different replication levels. J Proteome Res 6(12):4677–4683
Valcu CM, Reger K, Ebner J, Gorlach A (2012) Accounting for biological variation in differential display two-dimensional electrophoresis experiments. J Proteomics 75(12):3585–3591. https://doi.org/10.1016/j.jprot.2012.04.003
Voigt B, Schroeter R, Jurgen B, Albrecht D, Evers S, Bongaerts J, Maurer KH, Schweder T, Hecker M (2013) The response of Bacillus licheniformis to heat and ethanol stress and the role of the SigB regulon. Proteomics 13(14):2140–2161. https://doi.org/10.1002/pmic.201200297
Wolff S, Otto A, Albrecht D, Zeng JS, Büttner K, Glückmann M, Hecker M, Becher D (2006) Gel-free and gel-based proteomics in Bacillus subtilis: a comparative study. Mol Cell Proteomics Mcp 5(7):1183–1192
Xiao H, Zhang Y, Yong K, Kim S, Kim JJ, Kim KM, Yoshizawa J, Fan LY, Cao CX, Wong DTW (2016) Differential proteomic analysis of human saliva using tandem mass tags quantification for gastric cancer detection. Sci Rep 6:22165
Xie L, Liu W, Li Q, Chen S, Xu M, Huang Q, Zeng J, Zhou M, Xie J (2015) The first succinyl-proteome profiling of extensively drug resistant Mycobacterium tuberculosis revealed involvement of succinylation in cellular physiology. J Proteome Res 14(1):107
Zech H, Echtermeyer C, Wohlbrand L, Blasius B, Rabus R (2011) Biological versus technical variability in 2-D DIGE experiments with environmental bacteria. Proteomics 11(16):3380–3389. https://doi.org/10.1002/pmic.201100071
Zhang J, Fitzjames PC, Aronson AI (1993) Cloning and characterization of a cluster of genes encoding polypeptides present in the insoluble fraction of the spore coat of Bacillus subtilis. J Bacteriol 175(12):3757–3766
Zhou Y, Yu WB, Ye BC (2011) Variation of gene expression in Bacillus subtilis samples of fermentation replicates. Bioprocess Biosyst Eng 34(5):569–579
Acknowledgements
This research was supported by National Science Foundation of China (31401592).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic Supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, JQ., Yu, M., Zhou, Y. et al. Proteomic Analysis of Normal Expression Differences Exist in Bacillus Subtilis 168 Cultivation. Curr Microbiol 75, 803–810 (2018). https://doi.org/10.1007/s00284-018-1451-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-018-1451-y