Abstract
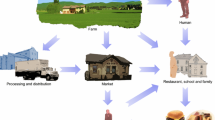
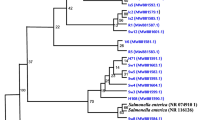
Campylobacter is one of the leading causes of foodborne travelers’ diarrhea worldwide. Although a large number cases of campylobacteriosis go undiagnosed or unreported, it is considered as the second most common foodborne illness in the USA affecting over 1.3 million individuals every year. Of various Campylobacter species, C. jejuni, C. coli, and C. lari have been accounted for causing more than 99% of human infections. Thus, there is a need to have efficient isolation method to protect public health on food safety and monitoring the burden of campylobacteriosis. Nevertheless, it is a challenging task as the exposure of environmental stress during isolation process makes Campylobacter species less culturable. Sixteen Campylobacter spp. were used to evaluate the current protocols used in Campylobacter isolation. For optimal recovery, a range of growth media (Bolton, Columbia, Muller Hinton, CVA Campy and mCCDA), incubation temperatures, and additional supplements (including antibiotics) were tested. Blood agars without antibiotics were sufficient for the initial recovery. Afterward, the isolates could grow on agars without any supplements, and in some cases growth was observed in the presence of antibiotics. Incubation at 37 °C was found to be the optimal temperature for the recovery and the growth of most species. Additionally, a food adulteration study was also carried out by artificially contaminating three food matrices that included egg, milk, and infant cereal, with two isolates of C. jejuni and C. coli. Results of this study should provide the insight for culturing and isolation of Campylobacter from food and other sources.
Similar content being viewed by others
References
Bolton FJ, Coates D (1983) Development of a blood-free Campylobacter medium—screening-tests on basal media and supplements, and the ability of selected supplements to facilitate aerotolerance. J Appl Bacteriol 54:115–125
Bolton FJ, Robertson L (1982) A selective medium for isolating Campylobacter-jejuni-coli. J Clin Pathol 35:462–467
Bolton FJ, Hutchinson DN, Coates D (1984) Blood-free selective medium for isolation of Campylobacter jejuni from feces. J Clin Microbiol 19:169–171
Casals C, Schellenberg D, Urassa H, Vargas M, Gasco J, Kahigwa E (2004) Etiology of diarrhea in children less than five years of Age. Am J Trop Med Hyg 70:536–539
Dasti JI, Tareen AM, Lugert R, Zautner AE, Gross U (2010) Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol 300:205–211
Davis L, DiRita V (2005) Growth and laboratory maintenance of Campylobacter jejuni. Curr Protoc Microbiol. doi:10.1002/9780471729259.mc08a01s10
Epps SV, Harvey RB, Hume ME, Phillips TD, Anderson RC, Nisbet DJ (2013) Foodborne Campylobacter: infections, metabolism, pathogenesis and reservoirs. Int J Environ Res Public Health 10:6292–6304
Fakruddin M, Mannan KSB, Andrews S (2013) Viable but nonculturable bacteria: food safety and public health perspective. ISRN Microbiol. doi:10.1155/2013/703813
FDA Bacteriological Analytical Manual (BAM). https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm072616.htm. Accessed Jan 6, 2017
Fitzgerald C, Nachamkin I (2015) Campylobacter and Arcobacter. In: Jorgensen J, Pfaller M, Carroll K, Funke G, Landry M, Richter S, Warnock D (eds) Manual of clinical microbiology, 11th edn. ASM Press, Washington, DC. doi:10.1128/9781555817381.ch56
Gardner TJ, Fitzgerald C, Xavier C, Klein R, Pruckler J, Stroika S, McLaughlin JB (2011) Outbreak of campylobacteriosis associated with consumption of raw peas. Clin Infect Dis 53:26–32
Gharst G, Oyarzabal OA, Hussain SK (2013) Review of current methodologies to isolate and identify Campylobacter spp. from foods. J Microbiol Methods 95:84–92
Guerry P (2007) Campylobacter flagella: not just for motility. Trends Microbiol 15:456–461
Gun-Munro J, Rennie RP, Thornley JH, Richardson HL, Hodge D, Lynch J (1987) Laboratory and clinical evaluation of isolation media for Campylobacter jejuni. J Clin Microbiol 25:2274–2277
Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM (2015) Global epidemiology of Campylobacter infection. Clin Microbiol Rev 28:687–720
Kabir MS, Hsieh YH, Simpson S, Kerdahi K, Sulaiman IM (2017) Evaluation of two standard and two chromogenic selective media for optimal growth and enumeration of isolates of 16 unique Bacillus species. J Food Prot 80:952–962
Komagamine T, Yuki N (2006) Ganglioside mimicry as a cause of Guillain-Barre syndrome. CNS Neurol Disord Drug Targets 5:391–400
Kuroki S, Saida T, Nukina M, Haruta T, Yoshioka M, Kobayashi Y, Nakanishi H (1993) Campylobacter jejuni strains from patients with Guillain-Barre syndrome belong mostly to Penner serogroup 19 and contain beta-N-acetylglucosamine residues. Ann Neurol 33:243–247
Li L, Mendis N, Trigui H, Oliver JD, Faucher SP (2014) The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol 5:258–278
Mace S, Haddad N, Zagorec M, Tresse O (2015) Influence of measurement and control of microaerobic gaseous atmospheres in methods for Campylobacter growth studies. Food Microbiol 52:169–176
Magajna BA, Schraft H (2015) Campylobacter jejuni biofilm cells become viable but non-culturable (VBNC) in low nutrient conditions at 4°C more quickly than their planktonic counterparts. Food Control 50:45–50
Magajna BA, Schraft H (2016) Evaluation of propidium monoazide and quantitative PCR to quantify viable Campylobacter jejuni biofilm and planktonic cells in log phase and in a viable but nonculturable state. J Food Prot 78:1303–1311
Man SM (2011) The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol 8:669–685
Moore JE, Corcoran D, Dooley JS, Fanning S, Lucey B, Matsuda M, McDowell DA, Megraud F, Millar BC, O’Mahony R, O’Riordan L, O’Rourke M, Rao JR, Rooney PJ, Sails A, Whyte P (2005) Campylobacter. Vet Res 36:351–382
Ng LK, Stiles ME, Taylor DE (1985) Comparison of basal media for culturing Campylobacter jejuni and Campylobacter coli. J Clin Microbiol 21:226–230
Oh E, McMullen L, Jeon B (2015) Impact of oxidative stress defense on bacterial survival and morphological change in Campylobacter jejuni under aerobic conditions. Front Microbiol 6:295–303
Penner JL (1988) The genus Campylobacter: a decade of progress. Clin Microbiol Rev 1:157–172
Peterson MC (1994) Clinical aspects of Campylobacter jejuni infections in adults. West J Med 161:148–152
Portner DC, Leuschner RG, Murray BS (2007) Optimising the viability during storage of freeze-dried cell preparations of Campylobacter jejuni. Cryobiology 54:265–270
Ramamurthy T, Ghosh A, Pazhani GP, Shinoda S (2014) Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria. Front Public Health 2:103–112
Rodgers JD, Clifton-Hadley FA, Marin C, Vidal AB (2010) An evaluation of survival and detection of Campylobacter jejuni and C. coli in broiler caecal contents using culture-based methods. J Appl Microbiol 109:1244–1252
Sahin O, Kobalka P, Zhang Q (2003) Detection and survival of Campylobacter in chicken eggs. J Appl Microbiol 95:1070–1079
Scallan E, Hoekstra RM, Angulo FG, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM (2011) Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15
Tallent SM, Kotewicz KM, Strain EA, Bennett RW (2012) Efficient isolation and identification of Bacillus cereus group. J AOAC Int 95:446–451
Teramura H, Iwasaki M, Ogihara H (2015) Development of a novel chromogenic medium for improved Campylobacter detection from poultry samples. J Food Prot 78:1750–1755
Vandamme P, Falsen E, Rossau R, Hoste B, Segers P, Tytgat R, De Ley J (1991) Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int J Syst Bacteriol 41:88–103
Vondrakova L, Pazlarova J, Demnerova K (2014) Detection, identification and quantification of Campylobacter jejuni, coli and lari in food matrices all at once using multiplex qPCR. Gut Pathogens 6:12–21
Acknowledgements
This study was supported by funding from the FDA Commissioner’s Fellowship Program. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclaimer
The findings and conclusions made in this manuscript are those of the authors and do not necessarily represent the views or official position of U.S. Food and Drug Administration (FDA) or U.S. Department of Health and Human Services (DHHS). The names of vendors or manufacturers are provided as examples of available product sources; inclusion does not imply endorsement of the vendors, manufacturers, or products by FDA or DHHS.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hsieh, YH., Simpson, S., Kerdahi, K. et al. A Comparative Evaluation Study of Growth Conditions for Culturing the Isolates of Campylobacter spp.. Curr Microbiol 75, 71–78 (2018). https://doi.org/10.1007/s00284-017-1351-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1351-6