Abstract
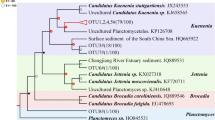
The distribution of anammox bacteria in rhizosphere sediments of cattail (Typha orientalis) at different phenological stages was investigated. Results showed that the number of 16S rRNA gene copies of the anammox bacteria was considerably higher in the rhizosphere sediment than in the nonrhizosphere sediment and control sediment. The abundances of the anammox bacteria exhibited striking temporal variations in the three different cattail phenological stages. In addition, the Chao1 and Shannon H indexes of the anammox bacteria in cattail rhizosphere sediments had evident spatial and temporal variations at different phenological stages. Four anammox genera (Brocadia, Kuenenia, Jettenia, and a new cluster) were detected and had proportions of 34.18, 45.57, 0.63, and 19.62%, respectively. The CCA analysis results indicated that Cu, TN, Pb, and Zn were pivotal factors that affect anammox bacteria composition. The PCoA analysis results indicated that the community structure at the rhizosphere and nonrhizosphere sediments collected on July was relatively specific and was different from sediments collected on other months, suggesting that cattail can influence the community structures of the anammox bacteria at the maturity stage.





Similar content being viewed by others
References
Amano T, Yoshinaga I, Okada K, Yamagishi T, Ueda S, Obuchi A, Sako Y, Suwa Y (2007) Detection of anammox activity and diversity of anammox bacteria-related 16S rRNA genes in coastal marine sediment in Japan. Microbes Environ 22:232–242
Armstrong W (1978) Root aeration in the wetland condition. In: Hook DE, Crawford RMM (eds) Plant Life in Anaerobic Environment. Ann Arbor Science Publisher, Ann Arbor, pp 267–297
Arrigo KR (2005) Marine microorganisms and global nutrient cycles. Nature 437:349–355
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266
Bodelier PLE, Libochant JA, Blom CWPM, Laanbroek HJ (1996) Dynamics of nitrification and denitrification in root-oxygenated sediments and adaptation of ammonia-oxidizing bacteria to low-oxygen or anoxic habitat. Appl Environ Microbiol 62:4100–4107
Chen WY, Chen ZH, He QF, Wang XY, Wang CR, Chen DF, Lai ZL (2007) Root growth of wetland plants with different root types. Acta Ecol Sin 27:450–457
Chu JY, Zhang JP, Zhou XH, Liu B, Li YM (2015) A comparison of anammox bacteria abundance and community structures in three different emerged plants-related sediments. Curr Microbiol 71:421–427
Dang HY, Zhou HX, Zhang ZN, Yu ZS, Hua E, Liu XS, Jiao NZ (2013) Molecular detection of Candidatus Scalindua pacifica and environmental responses of sediment anammox bacteria community in the Bohai Sea, China. PLoS ONE 8(4):e61330
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 12:1135–1142
Gao YZ, Ren LL, Ling WT, Gong SS, Sun BQ, Zhang Y (2010) Desorption of phenanthrene and pyrene in soils by root exudates. Bioresour Technol 101:1159–1165
Gruber N, Galloway JN (2008) An Earth-system perspective of the global nitrogen cycle. Nature 451:293–296
Hamady M, Lozupone C, Knight R (2009) Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J 4:17–27
Han HY, Li ZK (2016) Effects of macrophyte-associated nitrogen cycling bacteria on ANAMMOX and denitrification in river sediments in the Taihu Lake region of China. Ecol Eng 93:82–90
Han P, Gu JD (2013) More refined diversity of anammox bacteria recovered and distribution in different ecosystems. Appl Microbiol Biotechnol 97:3653–3663
Hartmann A, Rothballer M, Schmid M (2008) Lorenz Hiltner, A pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 312:7–14
Herrmann M, Saunders AM, Schramm A (2009) Effect of lake trophic status and rooted macrophytes on community composition and abundance of ammonia- oxidizing prokaryotes in freshwater sediments. Appl Environ Microbiol 75:3127–3136
He YL, Tao WD, Wang ZY, Shayya W (2012) Effects of pH and seasonal temperature variation on simultaneous partial nitrification and anammox in free-water surface wetlands. J Environ Manag 110:103–109
Hou LJ, Zheng YL, Liu M, Gong J, Zhang XL, Yin GY, You LL (2013) Anaerobic ammonium oxidation (anammox) bacteria diversity, abundance, and activity in marsh sediments of the Yangtze Estuary. J Geophys Res 118:1237–1246
Hu BL, Shen LD, Zheng P, Hu AH, Chen TT, Cai C, Liu S, Lou LP (2012) Distribution and diversity of anaerobic ammonium-oxidizing bacteria in the sediments of the Qiantang River. Riverem. Env Microbiol Rep 5:540–547
Humbert S, Tarnawski S, Fromin N, Mallet MP, Aragno M, Zopfi J (2010) Molecular detection of anammox bacteria in terrestrial ecosystems: distribution and diversity. ISME J 4:450–454
Jin RC, Yang GF, Yu JJ, Zheng P (2012) The inhibition of the anammox process: a review. Chem Eng J 197:67–79
Kuypers MMM, Lavik G, Woebken D, Schmid M, Fuchs BM, Amann R, Jørgensen BB, Jetten MSM, Hayes JM (2005) Massive nitro- gen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc Natl Acad Sci 102:6478–6483
Ladygina N, Hedlund K (2010) Plant species influence microbial diversity and carbon allocation in the rhizosphere. Soil Biol Biochem 42:162–168
Li H, Yang XR, Weng BS, Su JQ, Nie SA, Gilbert JA, Zhu YG (2016) The phenological stage of rice growth determines anaerobic ammonium oxidation activity in rhizosphere soil. Soil Biol Biochem 100:59–65
Liu B, Li YM, Zhang JP, Zhou XH, Wu CD (2014) Abundance and diversity of ammonia-oxidizing microorganisms in the sediments of Jinshan Lake. Curr Microbiol 69:751–757
Liu ZH, Huang SB, Sun GP, Xu ZC, Xu MY (2011) Diversity and abundance of ammonia-oxidizing archaea in the Dongjiang River, China. Microbiol Res 166:337–345
Lugtenberg BJJ, Chin-A-Woeng TFC, Bloemberg GV (2002) Microbe-plant in-teractions: principles and mechanisms. Anton Leeuw Int J G 81:373–383
Mulder A, Graaf AAVD, Robertson LA, Kuenen JG (1995) Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiol Ecol 16:177–184
Nie SA, Li H, Yang XR, Zhang ZJ, Weng BS, Huang FY, Zhu GB, Zhu YG (2015) Nitrogen loss by anaerobic oxidation of ammonium in rice rhizosphere. ISME J 9:2059–2067
Nicholls JC, Trimmer M (2009) Widespread occurrence of the anammox reaction in estuarine sediments. Aquat Microb Ecol 55:105–113
Ottosen LDM, Risgaard-Petersen N, Nielsen LP (1999) Direct and indirect measurements of nitrification and denitrification in the rhizosphere of aquatic macrophytes. Aquat Microb Ecol 19:81–91
Reddy KR, Patrick WH, Lindau CW (1989) Nitrification-denitrification at the plant root-sediment interface in wetlands. Limnol Oceanogr 34:1004–1013
Risgaard-Petersen N, Jensen K (1997) Nitrification and denitrification in the rhi- zosphere of the aquatic macrophyte Lobelia dortmanna L. Limnol Oceanogr 42:529–537
Rysgaard S, Glud RN, Risgaard-Petersen N, Dalsgaard T (2004) Denitrification and anammox activity in Arctic marine sediments. Limnol Oceanogr 49:1493–1502
Schloss PD, Handelsman J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71:1501–1506
Schmid M, Schmitz-Esser S, Jetten M, Wagner M (2011) 16S-23S rDNA intergenic spacer and 23S rDNA of anaerobic ammonium-oxidizing bacteria: implications for phylogeny and in situ detection. Environ Microbiol 3:450–459
Schmid M, Twachtmann U, Klein M, Strous M, Juretschko S, Jetten M, Metzger JW, Schleifer KH, Wagner M (2000) Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst Appl Microbiol 2723:93–106
Shao SD, Luan XW, Dang HY, Zhou HX, Zhao YK, Liu HT, Zhang YB, Dai LQ, Ye Y (2014) Martin G. Klotz. Deep-sea methane seep sediments in the Okhotsk Sea sustain diverse and abundant anammox bacteria. FEMS Microbiol Ecol 87:503–516
Shen LD, Liu S, Lou LP, Liu WP, Xu XY, Zheng P, Hu BL (2013) Broad dis- tribution of diverse anaerobic ammonium-oxidizing bacteria in Chinese agricultural soils. Appl Environ Microbiol 79:6167–6172
Shen LD, Liu S, Huang Q, Lian X, He ZF, Geng S, Jin RC, He YF, Lou LP, Xu XY, Zheng P, Hu BL (2014) Evidence for the co-occurrence of nitrite-dependent anaerobic ammonium and methane oxidation processes in a flooded paddy field. Appl Environ Microbiol 80:7611–7619
Shen LD, Wu HS, Gao ZQ, Xu XH, Chen TX, Liu S, Cheng HX (2015) Occurrence and importance of anaerobic ammonium-oxidising bacteria in vegetable soils. Appl Microbiol Biotechnol 99:5709–5718
Singh DK, Kumar S (2008) Nitrate reductase, arginine deaminase, urease and dehydrogenases activities in natural soil (ridge with forest) and in cotton soil after cetamiprid treatments. Chemosphere 71:412–418
Srivastava J, Gupta A, Chandra H (2008) Managing water quality with aquatic macrophytes. Rev Environ Sci Biotechol 7:255–266
Strous M, Heijnen J, Kuenen J, Jetten M (1998) The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl Microbiol Biotechnol 50:589–596
Sun W, Xu MY, Wu WM, Guo J, Xia CY (2014) Molecular diversity and distribution of anammox community in sediments of the Dongjiang River, a drinking water source of Hong Kong. J Appl Microbiol 116:464–476
van der Star WRL, Miclea AI, van Dongen UGJM, Muyzer G, Picioreanu C, van Loosdrecht MCM (2008) The membrane bioreactor: a novel tool to grow anammox bacteria as free cells. Biotechnol Bioeng 101:286–294
Waki M, Yasuda T, Suzuki K, Komada M, Abe K (2015) Distribution of anammox bacteria in a free-water-surface constructed wetland with wild rice (Zizania latifolia). Ecol Eng 81:165–172
Wang L, Li T (2011) Anaerobic ammonium oxidation in constructed wetlands with bio-contact oxidation as pretreatment. Ecol Eng 37:1225–1230
Wang J, Kang J (2005) The characteristics of anaerobic ammonium oxidation (ANAMMOX) by granular sludge from an EGSB reactor. Process Biochem 40:1973–1978
Wang YF, Feng YY, Ma XJ, Gu JD (2013) Seasonal dynamics of ammonia/ammonium-oxidizing prokaryotes in oxic and anoxic wetland sediments of subtropical coastal mangrove. Appl Microbiol Biotechnol 97:7919–7934
Wang J, Gu JD (2013) Dominance of Candidatus Scalindua species in anammox community revealed in soils with different duration of rice paddy cultivation in Northeast China. Appl Microbiol Biotechnol 99:1785–1798
Wang YF, Gu JD (2013) Higher diversity of ammonia/ammonium-oxidizing prokaryotes in constructed freshwater wetland than natural coastal marine wetland. Appl Microbiol Biotechnol 97:7015–7033
Wu QL, Zwart G, Wu JF, Kamstvan Agterveld MP, Liu SJ, Hahn MW (2007) Submersed macrophytes play a key role in structuring bacterioplankton community composition in the large, shallow, subtropical Taihu Lake, China. Environ Microbiol 9:2765–2774
Yang XR, Li H, Nie SA, Su JQ, Weng BS, Zhu GB, Yao HY, Gilbert JA, Zhu YG (2015) Potential contribution of anammox to nitrogen loss from paddy soils in Southern China. Appl Environ Microbiol 81:938–947
Zhao DY, Luo J, Zeng J, Wang M, Yan WM, Huang R, Wu QL (2014) Effects of submerged macrophytes on the abundance and community composition of ammonia-oxidizing prokaryotes in a eutrophic lake. Environ Sci Pollut Res 21:389–398
Zeng J, Zhao DY, Huang R, Wu QL (2012) Abundance and community composi- tion of ammonia-oxidizing archaea and bacteria in two different zones of Lake Taihu. Can J Microbiol 58:1018–1026
Zheng YL, Hou LJ, Liu M, Yin GY, Gao J, Jiang XF, Lin XB, Li XF, Yu CD, Wang R (2016) Community composition and activity of anaerobic ammonium oxidation bacteria in the rhizosphere of salt-marsh grass Spartina alterniflora. Appl Microbiol Biotechnol 100:8203–8212
Zhu GB, Wang SY, Wang Y, Wang CX, Risgaard-Petersen N, Jetten MSM, Yin CQ (2011) Anaerobic ammonia oxidation in a fertilized paddy soil. ISME J 5:1905–1912
Zhu GB, Wang SY, Wang WD, Wang Y, Zhou LL, Jiang B, Op den Camp HJM, Risgaard-Petersen N, Schwark L, Peng YZ (2013) Hotspots of anaerobic ammonium oxidation at land- freshwater interfaces. Nat Geosci 6:103–107
Zhou XH, Zhang JP, Li YM, Liu B, Chu JY, Wang MY, He ZL (2016) Distribution characteristics of ammonia oxidizing microorganisms in rhizosphere sediments of cattail. Ecol Eng 88:99–111
Zhou XH, Li YM, Zhang JP, Liu B, Wang MY, Zhou YW, Lin ZJ, He ZL (2016) Diversity, abundance and community structure of ammonia-oxidizing archaea and bacteria in riparian sediment of Zhenjiang ancient canal. Ecol Eng 90:447–458
Acknowledgements
This study was supported by the Jiangsu province water conservancy science and technology project (Grant # 2016050), and Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, X., Zhang, J. & Wen, C. Community Composition and Abundance of Anammox Bacteria in Cattail Rhizosphere Sediments at Three Phenological Stages. Curr Microbiol 74, 1349–1357 (2017). https://doi.org/10.1007/s00284-017-1324-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1324-9