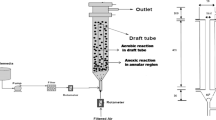
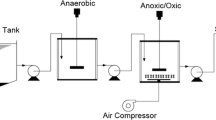
Abstract
In this study, the effect of operational conditions on biofilm development and nitrification in moving-bed biofilm reactor (MBBR) was investigated. The reactor was operated in a continuously fed regime during 170 days and with theoretical hydraulic retention time of 7 h, respectively. The presence of chemical oxygen demand (COD) increased the time required to form stable nitrifying. Subsequent stepwise increase of influent COD caused an increment in total polysaccharide (PS) and protein (PN) content, which was accompanied by an attachment of the biofilm, as shown by atomic force microscope (AFM). PS and PN concentrations proved to be good indicators of biomass development and attachment in MBBR system. Reactor was operated and water quality was characterized before and after treatment. Parameters including pH, 5-day biochemical oxygen demand (BOD5), total suspended solids (TSS) (COD), PN, PS, and fecal bacteria in both raw and treated wastewater were monitored during the treatment. The removal rates of ammonium-nitrogen (NH4 +-N), BOD5, COD, and TSS are 95, 67.5, 69.2, and 73.33 %, respectively. The average bacterial reduction between the inlet and the outlet was of the order of 5 ± 1 logarithmic units for fecal coliforms. AFM showed that distinct biofilm and extracellular polymeric substances were formed in biofilm was thicker in the 70 days than in the 30 days. These results showed that the consumption rate for each substrate increased parabolically with biofilm thickness due to the increased amount of biomass Thus, MBBR can serve as a promising technology for wastewater treatment and can be scaled up for small communities in the developing countries.






Similar content being viewed by others
References
Adav SS, Lee DJ (2008) Extraction of extracellular polymeric substances from aerobic granule with compact interior structure. J Hazard Mater 154(1–3):1120–1126
AFNOR (1992) Mise en œuvre des dispositifs d’assainissement autonome. Normalisation Française, DTU 64(1):36
Alves CF, Melo LF, Vieira MJ (2002) Influence of the medium composition on the characteristics of a denitrifying biofilm formed by Alcaligenes denitrificans in a fluidized bed reactor. Process Biochem 37:837–845
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Chem 72:248–254
Braga PC, Ricci D (1998) Atomic force microscopy: application to investigation of Escherichia coli morphology before and after exposure to cefodizime. Antimicrob Agents Chemother 42(1):18–22
Brenner A, van der Steen P, Oron G, Shabtai Y (2000) The effect of environmental conditions on faecal coliform decay in post-treatment of UASB reactor effluent. Water Sci Technol 42:111–118
Carles P, Barth FS (2014) Structure, composition, and strength of nitrifying membrane-aerated biofilms. Water Res 57:151–161
Chen MY, Lee DJ, Tay JH (2007) Distribution of extracellular polymeric substances in aerobic granules. Appl Microbiol Biotechnol 73(6):1463–1469
Chu W, Gao N, Deng Y, Templeton MR, Yin D (2011) Impacts of drinking water pretreatments on the formation of nitrogenous disinfection by-products. Bioresour Technol 102(24):11161–11166
Denkhaus E, Meisen S, Telgheder U, Wingender J (2007) Chemical and physical methods for characterisation of biofilms. Microchim Acta 158:1–27
Dubois M, Gilles KA, Hamilton PA, Smith F (1956) Colourimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
García-Mesa JJ, Poyatos JM, Delgado-Ramos F, Muñio MM, Osorio F, Hontoria E (2010) Water quality characterization in real biofilm wastewater treatment systems by particle size distribution. Bioresour Technol 101(21):8038–8045
Golam HS, Kaan Y, Amir HM, Mansur Z (2013) Post-treatment of secondary wastewater treatment plant effluent using a two-stage fluidized bed bioreactor system. J Environ Sci Health. doi:10.1186/2052-336X-11-10
Gürkan S, Jan W, Henri S, Ingmar N (2008) Dynamic model development and validation for a nitrifying moving bed biofilter: effect of temperature and influent load on the performance. Process Biochem 43:384–397
Jayamohan SJ, Ohgaki S, Hanaki K (1988) Effect of DO on kinetic of nitrification. J Water Supply Res T6:141–150
Kouki S, M’hiri F, Saidi N, Belaïd S, Hassen A (2009) Performances of a constructed wetland treating domestic wastewaters during a macrophytes life cycle. Desalination 246:452–467
Kristi B, Taylor MW, Turner SJ (2013) Successional development of biofilms in moving bed biofilm reactor (MBBR) systems treating municipal wastewater. Appl Microbiol Biotechnol 98:1429–1440
Liu Y, Tay JH (2002) The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Res 36:1653–1665
Mobarry BK, Wagner M, Urbain V, Rittmann B, Stahl D (1996) Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Appl Environ Microbiol 62:2156–2162
Qin L, Tay J-H, Liu Y (2004) Selection pressure is a driving force of aerobic granulation in sequencing batch reactors. Process Biochem 39(5):579–584
Ribeiro R, Varesche MBA, Foresti E, Zaiat M (2005) Influence of the carbon source on the anaerobic biomass adhesion on polyurethane foam matrices. J Environ Manag 74:187–194
Rodier J (1978) L’analyse de l’eau. Eaux naturelles, eaux résiduaires, eaux de mer, 6ème édition. Dunod, Paris
Tijhuis L, Huisman JL, Hekkelman HD, van Loosdrecht MC, Heijnen JJ (1995) Formation of biofilms on small suspended particles in airlift reactors. Biotechnol Bioeng 47(5):585–595
Van Dongen U, Jetten MS, van Loosdrecht MC (2001) The SHARON-Anammox process for treatment of ammonium rich wastewater. Water Sci Technol 44(1):153–160
Van Kempen R, Mulder JW, Uijterlinde CA, Loosdrecht MC (2001) Overview: full scale experience of the SHARON process for treatment of rejection water of digested sludge dewatering. Water Sci Technol 44(1):145–152
Xie SG, Tang XY, Wu WZ, Wen DH, Wang ZS (2005) Biological pretreatment of Yellow River water. J Environ Sci 17(4):557–561
Yang SF, Tay JH, Liu Y (2004) Inhibition of free ammonia to the formation of aerobic granules. J Biochem Eng 17:41–48
Yathavamoorthi R, Surendraraj A, Farvin KHS (2010) Enteric bacteria and water quality of freshwater prawn Macrobrachium rosenbergii (De Man) in culture environment from Kerala. J Fish Aquat Sci India 5:282–292
Ye F, Ye Y, Li Y (2011) Effect of C/N ratio on extracellular polymeric substances (EPS) and physicochemical properties of activated sludge flocs. J Hazard Mater 188:37–43
Zhang H, He Y, Jiang T, Yang F (2011) Research on characteristics of aerobic granules treating petrochemical wastewater by acclimation and co-metabolism methods. Desalination 279(1–3):69–74
Acknowledgments
This work was one of the outputs Research Programme Contract (2011–2013) under the title “Monitoring and Treatment of domestic wastewater Pollution nitrogen,” financed by Tunisian Ministry for Higher Education, Scientific Research and Technology. Thanks for experimental WWT staff especially for Nesrine khelifi and Mondher Zairi for their help during the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Houda, N., Abdelwaheb, C., Asma, B.R. et al. Tertiary Nitrification Using Moving-Bed Biofilm Reactor: A Case Study in Tunisia. Curr Microbiol 70, 602–609 (2015). https://doi.org/10.1007/s00284-014-0756-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0756-8