Abstract
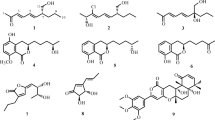
Endophytic fungi are ubiquitous in the plant kingdom and they produce a variety of secondary metabolites to protect plant communities and to show some potential for human use. However, secondary metabolites produced by endophytic fungi in the medicinal plant Curcuma wenyujin are sparsely explored and characterized. The aim of this study was to characterize the secondary metabolites of an active endophytic fungus. M7226, the mutant counterpart of endophytic fungus EZG0807 previously isolated from the root of C. wenyujin, was as a target strain. After fermentation, the secondary metabolites were purified using a series of purification methods including thin layer chromatography, column chromatography with silica, ODS-C18, Sephadex LH-20, and macroporous resin, and were analyzed using multiple pieces of data (UV, IR, MS, and NMR). Five compounds were isolated and identified as curcumin, cinnamic acid, 1,4-dihydroxyanthraquinone, gibberellic acid, and kaempferol. Interestingly, curcumin, one of the main active ingredients of C. wenyujin, was isolated as a secondary metabolite from a fungal endophyte for the first time.


Similar content being viewed by others
References
Cheng MJ, Wu MD, Hsieh SY et al (2011) Secondary metabolites from the endophytic fungus Annulohypoxylon boveri var. microspora BCRC 34012. Chem Nat Compd 47:536–540
Dhananjeyan MR, Milev YP, Kron MA et al (2005) Synthesis and activity of substituted anthraquinones against a human filarial parasite, Brugia malayi. J Med Chem 48:2822–2830
Ding YL, Xu AX (2005) The research on antitumor activity of Curcuma and its effective constitutes. J Chin Med Mater 28:152–156
Gaudreau C, Gilbert H (1997) Comparison of disc diffusion and agar dilution methods for antibiotic susceptibility testing of Campylobacter jejuni subsp. jejuni and Campylobacter coli. J Antimicrob Chemother 39:707–712
Ge HM, Tan RX (2009) Symbionts, an important source of new bioactive natural products. Pro Chem 21:30–46
Heinig U, Scholz S, Jennewein S (2013) Getting to the bottom of Taxol biosynthesis by fungi. Fungal Divers 60:161–170
Liang YC, Huang YT, Tsai SH et al (1999) Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis 20:1945–1952
Lu JR, Kang L, Xin CW et al (2007) Synthesis, characterization and properties of chloro-1,4-dihydroxyanthraquinones. J Tianjin Univ 40:1337–1341
Meazza G, Dayan FE, Wedge DE (2003) Activity of quinones on collectotrichum species. J Agr Food Chem 51:3824–3828
National Pharmacopoeia Committee (2005) Herbs and pieces. The Chinese Pharmacopoeia, 1st edn. Chemical Industry Press, Beijing, pp 194–195
National Pharmacopoeia Committee (2005) Plant oils and extracts. The Chinese Pharmacopoeia, 1st edn. Chemical Industry Press, Beijing, p 279
Scannerini S, Fusconi A, Mucciarelli M (2004) The effect of endophytic fungi on host plant morphogenesis. In: Seckback J (ed) Symbiosis: mechanisms and model systems. Kluwer Academic Publishers, New York, pp 427–447
Smith D, McCluskey K, Stackebrandt E (2014) Investment into the future of microbial resources: culture collection funding models and BRC business plans for biological resource centres. Springerplus 3:81
Stierle A, Strobel G, Stierle D (1993) Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 260:214–216
Strobel GA (2002) Rainforest endophytes and bioactive products. Crit Rev Biotechnol 22:315–333
Sung BK, Kim MK, Lee WH et al (2004) Growth responses of Cassia obtusifolia toward human intestinal bacteria. Fitoterapia 75:505–509
Tejesvi MV, Kini KR, Prakash HS et al (2007) Genetic diversity and antifungal activity of species of Pestalotiopsis isolated as endophytes from medicinal plants. Fungal Divers 24:37–54
Wei LH (2011) Studies on quality and infrared spectrum characteristics of Curcama kwangsinesis. Dissertation, Guangxi University, Guangxi, China
Wells JE, Berry ED, Varel VH (2005) Effects of common forage phenolic acids on Escherichia coli O157:H7 viability in bovine feces. Appl Environ Microbiol 71:7974–7979
Xiao YP, Chen JJ, Zhang YH et al (2004) Studies on the chemical constituents of Fusarium sp. from seagrass endophytic fungus. Chin J Mar Drugs 23:11–13
Xuan Q, Zhang QL, Yang J et al (2011) β-Elemene from Curcuma zedoaria endophytic fungus. Nat Prod Res Dev 23:473–475
Yan JF, Qi NB, Wang SP et al (2013) Identification and biological characteristics of an active endophytic fungus EZG0807. China Biotechnol 33:51–58
Yang SX (2011) Studies on secondary metabolites and their bioactivities of endophytic fungi from Melia Azedarach and Actinomycete from animals. Dissertation, Northwest University of Science and Technology, Shanxi, China
Yang X, Summerhurst DK, Koval SF et al (2001) Isolation of an antimicrobial compound from Impatiens balsamina L. using bioassay-guided fraction. Phytother Res 15:676–680
Yohei S, Hirozo G, Chihiro T et al (2003) Effects of curcuma drugs on vasomotion in isolated rat aorta. Biol Pharm Bull 26:1135–1143
Yu DQ, Yang JS (1999) Analytical chemistry handbook, vol VII. Chemistey industry Press, Beijing
Zhao JH, Zhang YL, Wang LW et al (2012) Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J Microbiol Biotechnol 28:2107–2112
Acknowledgments
The authors sincerely thank Jianfeng Zhao for providing valuable suggestion for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, J., Qi, N., Wang, S. et al. Characterization of Secondary Metabolites of an Endophytic Fungus from Curcuma wenyujin . Curr Microbiol 69, 740–744 (2014). https://doi.org/10.1007/s00284-014-0647-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0647-z