Abstract
Over the last decade, cholera outbreaks have become common in some parts of Kenya. The most recent cholera outbreak occurred in Coastal and Lake Victoria region during January 2009 and May 2010, where a total of 11,769 cases and 274 deaths were reported by the Ministry of Public Health and Sanitation. The objective of this study is to isolate Vibrio cholerae bacteriophages from the environmental waters of the Lake Victoria region of Kenya with potential for use as a biocontrol for cholera outbreaks. Water samples from wells, ponds, sewage effluent, boreholes, rivers, and lakes of the Lake Victoria region of Kenya were enriched for 48 h at 37 °C in broth containing a an environmental strain of V. cholerae. Bacteriophages were isolated from 5 out of the 42 environmental water samples taken. Isolated phages produced tiny, round, and clear plaques suggesting that these phages were lytic to V. cholerae. Transmission electron microscope examination revealed that all the nine phages belonged to the family Myoviridae, with typical icosahedral heads, long contractile tails, and fibers. Head had an average diameter of 88.3 nm and tail of length and width 84.9 and 16.1 nm, respectively. Vibriophages isolated from the Lake Victoria region of Kenya have been characterized and the isolated phages may have a potential to be used as antibacterial agents to control pathogenic V. cholerae bacteria in water reservoirs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholera is a waterborne diarrheal disease that continues to plague the developing world [9, 18]. Transmission of cholera is predominantly through fecal-contaminated food and water; thus, it is usually a disease of developing countries or areas, where clean water supply and adequate sanitation are lacking. Over the last decade, there have been increasing cholera outbreaks in Kenya [8, 10, 15]. Since 2007, Kenya has also been experiencing outbreaks of cholera, dysentery, and other diarrheal diseases related to poor access to potable water and effective sanitation systems. Inspite of the recent progress of medical sciences, cholera still remains as a severe global threat in view of morbidity and mortality and is currently spreading to countries such as Zimbabwe and Mozambique and in Africa and other parts of the world [5]. Vibrio cholerae, are normal inhabitants of fresh and salt water, thus, an understanding of cholera requires an understanding of the bacteria’s role in the environment well as in the human host.
Phages infecting V. cholerae are well documented with earliest records of isolation of bacteriophages appearing in 1920s [17]. In V. cholerae bacteriophages were first reported by Siddiqui and Bhattacharyya [13]. A major emphasis was placed on phage typing of V. cholerae and phage therapy was proposed for treatment of cholera in the early years of cholera research. Vibriophages might be employed as biological control agents in cholera epidemic areas [6, 7]. Lytic bacteriophages specific to V. cholerae may limit the severity of cholera outbreaks by killing bacteria present in the reservoir and in infected individuals [19]. Phage predation of V. cholerae has been reported to be a factor that influences epidemics of cholera in Bangladesh and India. Studies have found out that as cholera epidemic progressed in the course of the study, more and more cholera patients excreted both V. cholerae and lytic phages (JSF4) [6, 7]. Indeed predation on pathogenic V. cholerae may be an important factor influencing the epidemic cycle on short time scales and may act to modify the duration and severity of cholera outbreaks. Adding relatively large numbers of phage to the reservoir at the time of bacterial bloom decreases the size of cholera epidemic [9].
The role of bacteriophages in the environment has been the subject of intense investigation over the past several years. The development of techniques to study natural viral populations in situ has progressed tremendously. Various aspects of bacteriophage ecology in nature-including abundance, role in microbial mortality and water column trophodynamics, viral decay rates, repair mechanisms and lysogeny are gradually being understood [16]. In this study, we isolated and characterized vibriophages from the waters of the Lake Victoria region of Kenya and assessed their lytic activity against V. cholerae strain.
Materials and Methods
Vibrio cholerae Strain
A V. cholerae strain (8675) previously isolated from the Lake Victoria region of Kenya between 2009 and 2010 was retrieved from −80 °C freezer and subcultured on Blood agar (Himedia Laboratories, Mumbai), Thiosulphate citrate bile salt sucrose agar (TCBS) (Scharlau chemie SA) and Mueller–Hinton Agar (Oxoid, Basingstoke, UK). Incubation was done at 37 °C for 24 h. Working cultures were taken from TCBS plates. For enrichment of V. cholerae strain, a colony from nutrient agar was emulsified in a bottle of alkaline peptone water (5–10 ml) and incubated for 6–8 h at 37 °C.
Phage Isolation: Direct Enrichment of Water Samples
Water samples from the Lake Victoria region of Kenya had been preserved in 500 ml sterile containers at 24 °C at the time of analysis. Aliquot (50 ml) of the undiluted water samples was added into 100 ml sterile double strength TSB then inoculated with 24 h cultures of V. cholerae (50 ml), Escherichia coli (15 ml) (control) grown in single strength TSB at 37 °C. Incubation was done for 48 h at 37 °C with constant agitation at 50 rpm in a water bath. After 48 h of incubation, 45 ml of the content was dispensed into falcon tubes and centrifuged at 2,500×g for 15 min. Using a sterile syringe, 3 ml of the supernatant was taken, filtered by 0.45 μm into sterile screw-capped bottles and assayed for phages. Approximately 1–2 ml lysate was obtained after filtering. For storage, ~0.5 ml chloroform was added to the lysate, carefully-shaken, labeled “phage enriched”, and stored at 4 °C.
Testing the Enrichment for Phages: Spot Assay
The V. cholerae and E. coli were grown in TSB at 37 °C for 24 h. 3 ml of molten double strength soft TSB agar (0.6 % w/v) held at 45 °C was then mixed with 500 or 200 μl cells of V. cholerae and E. coli, respectively, and overlaid on the surface of regular TSA plates supplemented with 1 mM CaCl2. After agar solidification, 10 μl of the “enriched phage” was spotted at the center of the plate, left to dry and incubated at 37 °C for 24 h. At the spot where the phage will have been deposited bacterial sensitivity to bacteriophages was determined by monitoring the development of clear zones. A negative control comprising of sterile broth (TSB) was spotted at the center of the plated TSB agar.
Plaque Assay
The V. cholerae and E. coli were grown for 24 h at 37 °C in TSB. 500 μl of the “phage enriched” sample was mixed with 500 μl 24 h cultures of V. cholerae or 200 μl of E. coli. The mixture was left for 2–3 min at room temperature. After that, 3 ml of double strength TSB molten soft agar (0.6 % w/v) was added, mixed well, and poured on the surface of regular TSA plates. Plates were allowed to gel, incubated for 24 h at 37 °C, and observed for phage plaques identified as zones of cell lysis. A TSB molten agar plate that did not receive the enriched phage was used as negative control. Sample was scored positive if there were plaques observed and negative if there were no plaques. If no plaques were observed, further enrichment was performed (up to three rounds).
Spot Test on Phage on a Bacterial Streak
A loopful of the host bacteria grown in single strength TSB at 37 °C for 24 h was streaked on the surface of the TSA agar plates, which had been supplemented with CaCl2. The fluid was allowed to dry and then 5 μl of the clarified enrichments was spotted on the streaks, incubated for 24 h at 37 °C, and zones of cell lysis were observed. A control was also set using TSB broth spotted on the bacterial streak. A sample was scored positive if there was a clear zone at the center, where the enriched lysate was deposited along the bacterial streak and negative if the bacterial streak was continuous.
Preparation of Phage Lysates
In this research work, two methods of phage purification were employed; purification using a sterile pipette to pick a single plaque and streaking on TSA plates [11].
Examination of V. cholerae Bacteriophages by Transmission Electron Microscopy
Electron microscopy observations were performed directly on phage lysates. Phages were then sedimented in a Beckman OPTIMATM TL ultracentrifuge with a Beckman TLA 110 rotor at 25,000g for 60 min followed by two washes in fresh 0.1 M ammonium acetate (pH 7.2). 5 μl of this suspension was added to hydrophilic freshly glow-discharged EM grids with carbon-coated Formvar film and left to settle for 30 s. Then, 5 μl of 3 % (w/v) ammonium molybdate was added for 10 s and dried off with Whatman N°1 filter paper. The grid was air dried and viewed on a Philips Spirit Biotwin transmission electron microscope at an acceleration voltage of 120 kV. Examples of phage particles morphologies observed were photographed for further examination.
Results and Discussion
Assays and Source of the Water Samples
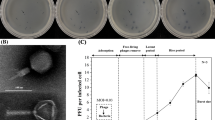
Table 1 shows the results of the three assays; plaque assay, spot assay, and streaking technique, sample code and the sources of these water samples for the water samples that were positive for at least one of the assays. The sources of these water samples included sewage, pond, lake, river, and unprotected well. A total of ten environmental water samples were positive for the spot assays. Out of these ten samples, only five were positive for plaque assay. Sample 103 showed the highest plaque assay with 36 pfu/ml, while sample 78 had the lowest; 16 pfu/ml. Plaques were not detected in sample 189b that traced its source from the sewage but were detected in the other three samples (103, 132, and 105) that were also from sewage effluent. These samples that were positive for plaque assay were also positive for spot assay and on a bacterial streak, as shown in Table 1. Samples 189A, 42, and 35 traced their sources from the Lake. In these three samples plaques were not detected but the other two assays were positive. Similar results were observed from sample 79 that was from unprotected well. All the other 32 water samples were negative for plaque assays.
The plaques obtained were all tiny, clear, and round. Complete lysis was therefore inferred. Plaque concentrations ranged from 16 to 36 pfu/ml. Previously, a total of 32 sewage samples were collected in a period of 8 months from Sarapui River in Rio [14]. The plaques of these phages were observed as clear and round with a diameter of 3–4 mm. The morphology of the phages was studied by negative staining, 034 phages showing a hexagonal head with a long tail and 06 phages had a similar form [14]. No comprehensive studies have been carried out to look for V. cholerae bacteriophages in fresh water environments in Kenya. To our knowledge, this is the first study to isolate and characterize vibriophages lytic to V. cholerae in particular, in the water bodies in and around the Lake Victoria region. It should be mentioned here that several earlier studies conducted elsewhere have reported on the relationship between environmental cholera phages and the seasonal epidemics of cholera [6, 7, 9]. Recent studies on prevalence of vibriophages and V. cholerae in the environment from two major rivers and a lake in Dhaka, where cholera outbreaks occur every year, a total of 221 water samples analyzed from January to November 2003. 114 samples contained either vibriophage or an epidemic V. cholerae whereas only 15 samples contained both. The plaques were clear or turbid. Clear signifying phages were lytic and highly virulent for V. cholerae and turbid indicating they were temperate.
Figure 1 shows a positive spot assay of a phage on a lawn of V. cholerae. At the center of the lawn where the lysate was spotted is a clear zone. This was indicative of the presence of lytic phages against V. cholerae.
Figure 2 shows a micrograph of the V. cholerae strain used. The length and width of this strain were 2,000 and 250 nm, respectively. This strain is known to persist in the environmental waters of the Lake region of Kenya [3]. After an outbreak of cholera subsides in the Lake region of Kenya, it has been suggested that V. cholerae becomes extinct in that locale, but that isolated pockets of disease linger elsewhere in the region. When the climate becomes favorable, V. cholerae re-emerges and spreads from the refuge. The regularity of these outbreaks indicates that V. cholerae might be frequently spread by travelers or that it is endemic in the area [3]. V. cholerae strain used as a host had been previously isolated from the same environmental waters in the same region. This was evidence that the host and the phages coexist in the same water reservoirs.
Morphology of the Phages
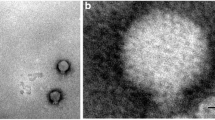
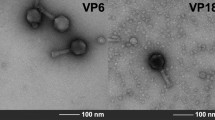
Out of the 42 environmental water samples analyzed in this study, nine bacteriophages lytic to environmental V. cholerae, were isolated. In all of these images, a neck was evident between the head and the tail of the phage and fibers were clearly seen. All the phages presented a long contractile tail except 14e which had a contracted tail. Based on these morphological characteristics, the phage was therefore assigned to Myoviridae family, order Caudovirales according to the International Committee on Taxonomy of Viruses (ICTV) classification [1, 2] (Fig. 3).
Transmission electron micrographs of Vibrio cholerae vibriophages isolated from the waters of the Lake Victoria region of Kenya. Bars 100 nm. Monographs 4a–4c were from the water sample 103 that traced its source from sewage effluent. Monographs 14a–14h were from water sample 174 that traced its source from a river. Vibriophages isolated morphology were examined by electron microscope
Lytic bacteriophage predation on pathogenic V. cholerae may be an important extrinsic factor influencing the epidemic cycle on short time scales and may act to modify the duration and severity of cholera outbreaks. Adding relatively large numbers of phage in the reservoir at the time of bacterial bloom decreases the size of cholera epidemic [9]. These findings suggested that these phages could be used as a biological control of the pathogenic V. cholerae in the environmental waters to control cholera in Kenya. Previous research showed that from 1928 to 1934; over a million Vibriophages were prepared and disseminated in specific study communities in India. This application was novel because for the first time, Vibriophages were disseminated into drinking water sources as a means of prophylaxis. The triennial death rates from cholera fell from 30 to 2 % per 10,000 in communities that were treated with phage [12]. Epidemiological and environmental observations of a cholera outbreak in Dhaka, Bangladesh, suggested that lytic bacteriophage specific for V. cholerae may limit the severity of cholera outbreak by killing bacteria in the reservoir and infected individuals [9]. If bacteriophages are present, persistently, or transiently, predation on V. cholerae in the reservoir can influence the course of cholera outbreaks. A high initial phage density of 108 would prevent ~3,000 cases of cholera compared with phage free outbreaks [9]. When the phage cannot maintain their densities and the fluctuations in V. cholerae densities are due to ecological processes such as resource limitations, externally introduced phage may still lead to reductions in outbreak severity. Experiments have shown that spiking water samples with phages only modestly reduces the titers of viable V. cholerae and only when phages are added at levels greater then 100 pfu/ml [6].
Phage therapy has also been used to fight cholera. The first therapy trial compared 244 untreated cholera patients with 219 patients who were treated with vibriophages: the untreated group had 20 % mortality rate whereas mortality in the treated group was 6.8 % [12].
The measurements of the seven bacteriophages are shown in Table 2. The average diameter of the head from point to point laterally was 88.3 nm.
The measurement of the phage tail length below the neck collar of seven phages was 84.9 nm. Measurement of the average tail width of the seven phages was 16.1 nm. These measurements show that these phages have similar dimensions and are within the known range of Caudovirales. Other previous characterized vibrio phages belong also to the same family of phages.
The vibriophage is also related to a previously characterized phage, JSF4 [6, 7]. Three vibriophages, AS1, AS2, and AS3 were isolated from the sewage and pond waters of the outskirts of Kolkata, phages AS1 and AS2 had hexagonal heads and contractile tails and were of the family of Myoviridae [4]. Other studies have showed that a V. cholerae bacteriophages isolated from the sea water collected from the coastal water of Lima, Peru, belonged to the family of Myoviridae [17].
In conclusion, this study demonstrated that the environmental waters of the Lake Victoria region of Kenya contained bacteriophages infective to V. cholerae. The bacteriophage which was presumptively identified as a member of Myoviridae family has potential as a biocontrol agent in cholera epidemic areas in Kenya.
References
Ackermann HW (2001) Frequency of morphological phage descriptions in the year 2000. Arch Virol 146:843–857
Ackermann HW (2003) Bacteriophage observations and evolution. Res Microbiol 154:245–251
Ahmed AM, Oundo J, Kariuki SM, Boga HI, Shanaz KS, Akhwale W, Omolo J, Anyangu SA, Mutonga D, Kareko D, Njeru M, Shan L, Breiman RF, Stine OS (2012) Molecular epidemiology of geographically dispersed Vibrio cholerae, Kenya, January 2009–May 2010. Emerg Infect Dis 18(6):925–931
Anindito S, Ghosh AN (2005) New Vibrio cholerae O1 biotype ElTor bacteriophages. J Virol 2:28
Das P, Mukherjee D (2012) Qualitative analysis of a cholera bacteriophage model. ISRN Biomath 2012. doi:10.5402/2012/621939
Faruque SM, Naser IB, Islam MJ, Faruque ASG, Ghosh AN, Nair GB, Sack DA, Mekalanos JJ (2005) Seasonal Epidemics of cholera inversely correlate with the prevalence of environmental cholera phages. Proc Natl Acad Sci USA 102:1702–1707
Faruque SM, Islam MJ, Ahmed QS, Faruque ASG, Sack DA, Nair GB, Mekalanos JJ (2005) Self-limiting nature of seasonal cholera epidemics: role of host-mediated amplification of phage. Proc Natl Acad Sci USA 102:6119–6124
Iijima Y, Oundo JO, Taga K, Saidi SM, Honda T (1995) Simultaneous outbreak due to Vibrio cholerae and Shigella dysentriae in Kenya. Lancet 345:69–70
Jensen MA, Faruque SM, Mekalanos JJ, Levin BR (2006) Modelling the role of bacteriophages in the control of cholera outbreaks. Proc Natl Acad Sci USA 103:4652–4657
Mugoya I, Kariuki S, Galgalo T, Njuguna C, Omollo J, Njoroge J, Kalani R, Nzioka C, Tetteh C, Bedno S, Breiman RF, Feikin DR (2008) Rapid spread of Vibrio cholerae 01 throughout Kenya, 2005. Am J Trop Med Hyg 78:527–533
Mullan WMA (2001) Isolation and purification of bacteriophages. http://www.dairyscience.info/index.php/isolation-and-purification-of-bacteriophages.html. Accessed 25 Jan 2012
Nelson EJ, Jason BH, Glenn JM Jr, Stephen BC, Andrew C (2009) Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol 7(10):693–702
Siddiqui KAI, Bhattacharyya FK (1987) Phage-induced change of toxigenesis in Vibrio cholerae. J Med Microbiol 23:331–334
Sarkar BL, Ghosh AN, Sen A, Rodrigues DP (2004) Newly isolated Vibrio cholerae non-O1, non-o139 phages. Emerg Infect Dis 10:754–756
Scrascia M, Maimone F, Mohamud KA, Materu SF, Grimont F, Grimont PAD, Pazzani C (2006) Clonal relationship among Vibrio cholerae 01 El Tor strains causing the largest cholera epidemic in Kenya in the late 1990s. J Clin Microbiol 44:3401–3404
Seed KD, Bodi KL, Kropinski AM, Ackermann HW, Calderwood SB (2011) Evidence of a dominant lineage of Vibrio cholerae-specific lytic bacteriophages shed by cholera patients over a 10-year period in Dhaka, Bangladesh. mBio 2(1):e00334. doi:10.1128/mBio.00334-10
Talledo M, Rivera IN, Lipp EK, Neale A, Karaolis D, Huq A, Colwell RR (2003) Characterization of a Vibrio cholerae phage isolated from the coastal water of Peru. Environ Microbiol 5(5):350–354
WHO (2002) Vibrio cholerae. In: Guidelines for drinking-water quality, 2nd edn. Addendum: microbiological agents in drinking water. World Health Organization, Geneva p 119–142
Zahid MSH, Udden SMN, Farugue ASG, Calderwood SB, Mekalanos J, Shah MF (2008) Effect of phage on the infectivity of Vibrio cholerae and emergence of genetic variants. Infect Immun 76:5266–5273
Acknowledgments
We acknowledge the Wellcome Trust Sanger Institute, Cambridge, UK, for TEM analysis of the Vibriophages as well as the Vibrio cholerae. KEMRI for providing the Vibrio cholerae isolate used in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Maina, A.N., Mwaura, F.B., Oyugi, J. et al. Characterization of Vibrio cholerae Bacteriophages Isolated from the Environmental Waters of the Lake Victoria Region of Kenya. Curr Microbiol 68, 64–70 (2014). https://doi.org/10.1007/s00284-013-0447-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-013-0447-x