Abstract
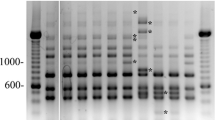
The aim of the present study was to perform molecular typing of Paenibacillus larvae (P. larvae) isolates from Bulgarian apiaries with repetitive element polymerase chain reaction (rep-PCR) using BOX A1R, MBO REP1, and ERIC primers. A total of 96 isolates collected from brood combs with clinical symptoms of American foulbrood originating from apiaries located in different geographical regions of Bulgaria, a reference strain P. larvae NBIMCC 8478 and 30 commercial honey samples with Bulgarian origin were included in the study. Rep-PCR fingerprinting analysis revealed two genotypes ab and AB of P. larvae isolates from brood combs and honey samples. A combination of genotypes ab/AB was detected in one apiary and honey sample. The prevailing genotype ab was found in 78.1 % of brood combs isolates as well as in the reference strain whereas genotype AB was determined in 21.9 % of isolates. The examination of honey samples confirmed the preponderance of ab genotype which was demonstrated in 20 of 30 samples analyzed. In conclusion, the genetic epidemiology of P. larvae revealed two genotypes—ab and AB for Bulgarian strains. Developed protocols for molecular typing of P. larvae are reliable and may be used to trace the source of infection.


Similar content being viewed by others
References
Alippi AM, Aguilar OM (1998) Characterization of isolates of Paenibacillus larvae subsp. larvae from diverse geographical origin by the polymerase chain reaction and BOX primers. J Invertebr Pathol 72:21–27
Alippi AM, López AC, Aguilar OM (2004) A PCR-based method that permits specific detection of Paenibacillus larvae subsp. larvae, the cause of American foulbrood of honey bees, at the subspecies level. Lett Appl Microbiol 39:25–33
Ashiralieva A, Genersch E (2006) Reclassification, genotypes and virulence of Paenibacillus larvae, the etiological agent of American foulbrood in honeybees–a review. Apidologie 37:411–420
Di Pinto A, Novello L, Terio V, Tantillo G (2011) ERIC-PCR genotyping of Paenibacillus larvae in southern italian honey and brood combs. Curr Microbiol 63:416–419
Djordjevic S, Ho-Shon M, Hornitzky M (1994) DNA restriction endonuclease profiles and typing of geographically diverse isolates of Bacillus larvae. J Apicult Res 33:95–103
Fries I, Camazine S (2001) Implications of horizontal and vertical pathogen transmission or honey bee epidemiology. Apidologie 32:199–214
Genersch E, Ashiralieva A, Fries I (2005) Strain and genotype-specific differences in virulence of Paenibacillus larvae subsp. larvae, a bacterial pathogen causing american foulbrood disease in honeybees. Appl Environ Microbiol 71:7551–7555
Genersch E, Forsgren E, Pentikäinen J et al (2006) Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. Int J System Evol Microbiol 56:501–511
Genersch E, Otten C (2003) The use of repetitive element PCR fingerprinting (rep-PCR) for genetic subtyping of German field isolates of Paenibacillus larvae subsp. larvae. Apidologie 34:195–206
Genersch E (2010) American foulbrood in honeybees and its causative agent, Paenibacillus larvae. J Invertebr Pathol 103:S10–S19
Hiett KL, Seal BS (2009) Use of repetitive element palindromic PCR (rep-PCR) for the epidemiologic discrimination of foodbornhe pathogens. Methods Mol Biol 551:49–58
Iurlina MO, Fritz R (2005) Characterization of microorganisms in Argentinian honeys from different sources. Int J Food Microbiol 105:297–304
Lindström A, Korpela S, Fries I (2008) Horizontal transmission of Paenibacillus larvae spores between honey bee (Apis mellifera) colonies through robbing. Apidologie 39:515–522
Loncaric I, Derakhshifar I, Oberlerchner JT, Köglberger H, Moosbeckhofer R (2009) Genetic diversity among isolates of Paenibacillus larvae from Austria. J Invertebr Pathol 100:44–46
Matheson A, Reid M (1992) Strategies for prevention and control of American foulbrood. Am Bee J 132:399–402
Neuendorf S, Hedtke K, Tangen G, Genersch E (2004) Biochemical characterization of different genotypes of Paenibacillus larvae subsp. larvae, a honey bee bacterial pathogen. Microbiology 150:2381–2390
Parvanov P, Russenova N, Dimov D (2006) Control of American foulbrood disease without antibiotic use. U Bee J 6:97–103
Pentikäinen J, Kalliainen E, Pelkonen S (2009) Molecular epidemiology of Paenibacillus larvae infection in Finland. Apidologie 40:73–81
Peters M, Kilwinski J, Beringhoff A, Reckling D, Genersch E (2006) American foulbrood of the honey bee: occurrence and distribution of different genotypes of Paenibacillus larvae in the administrative district of Arnsberg (North Rhine-Westphalia). J Vet Med B 53:100–104
Riley LW (2004) Molecular epidemiology of infectious diseases. ASM Press, Washington, USA, Principles and Practices
Rusenova NV, Parvanov P, Stanilova S (2013) Development of multiplex PCR for fast detection of Paenibacillus larvae in putrid masses and in isolated bacterial colonies. Appl Biochem Microbiol. doi:10.1134/S0003683813010171
Tobes R, Pareja E (2006) Bacterial repetitive extragenic palindromic sequences are DNA targets for insertion sequence elemets. BMC Genomics 7:62
Versalovic J, Schneider M, De Bruijn FJ, Lupski JR (1994) Genomic fingerprinting of bacteria using repetitive sequence based polymerase chain reaction. Methods Mol Cell Biol 5:25–40
Wu XY, Chin J, Ghalayini A, Hornitzky M (2005) Pulsed-field gel electrophoresis typing and oxytetracycline sensitivity of Paenibacillus larvae subsp. larvae isolates of Australian origin and those recovered from honey imported from Argentina. J Apicult Res 44:87–92
Acknowledgment
The research was funded by the projects 4/2009 and 4/2012 at Trakia university, Faculty of Veterinary Medicine, Stara Zagora, Bulgaria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rusenova, N., Parvanov, P. & Stanilova, S. Molecular Typing of Paenibacillus larvae Strains Isolated from Bulgarian Apiaries Based on Repetitive Element Polymerase Chain Reaction (Rep-PCR). Curr Microbiol 66, 573–577 (2013). https://doi.org/10.1007/s00284-013-0318-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-013-0318-5