Abstract
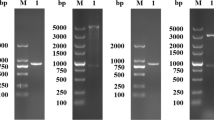
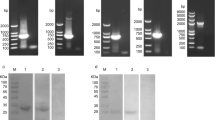
Cryptosporidium parvum, an intestinal apicomplexan parasite, is a significant cause of diarrheal diseases in both humans and animals. What is more, there is no promising strategy for controlling cryptosporidiosis. In this study, the P23 immunodominant surface protein of C. parvum sporozoites was stably expressed in the Lactobacillus casei Zhang strain and its immunogenicity was evaluated in a mouse model. The molecular weight (23 kDa) and immunogenicity of p23 gene expressed by L. casei Zhang were similar to that of the native P23 protein. Oral immunization with control L. casei Zhang and recombinant L. casei Zhang-p23 activated the mucosal immune system to elicit serum immunoglobulin G (IgG) and mucosal IgA in mice. Furthermore, the expression of cytokines such as IL-4, IL-6, and IFN-γ in splenocytes of mice was detected by real-time PCR after oral immunization. P23-specific immunocyte activation was also verified. These findings indicate that the live L. casei Zhang vector may be a new tool for the production of mucosal vaccines against cryptosporidiosis in animals.





Similar content being viewed by others
References
Adachi K, Kawana K, Yokoyama T et al (2010) Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine 28:2810–2817
Ajjampur SS, Sankaran P, Kang G (2008) Cryptosporidium species in HIV-infected individuals in India: an overview. Natl Med J India 21:178–184
Barakat FM, McDonald V, Di Santo JP, Korbel DS (2009) Roles for NK cells and an NK cell-independent source of intestinal gamma interferon for innate immunity to Cryptosporidium parvum infection. Infect Immun 77:5044–5049
Benitez AJ, McNair N, Mead JR (2009) Oral immunization with attenuated Salmonella enterica Serovar Typhimurium encoding Cryptosporidium parvum Cp23 and Cp40 antigens induces a specific immune response in mice. Clin Vaccine Immunol 16:1272–1278
Bernudez-Humaran LG, Cortes-Perez N, Le Loir Y et al (2004) An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J Med Microbiol 53:427–433
Campbell LD, Stewart JN, Mead JR (2002) Susceptibility to Cryptosporidium parvum infections in cytokine- and chemokine-receptor knockout mice. J Parasitol 88:1014–1016
de Graaf DC, De Coninck H, De Clercq C, Peeters JE (2002) Screening of the T- and B-cell antigenicity in neonatal calves of the His-tagged Cryptosporidium parvum antigens CP15, CP15/60, P23 and TRAP-C1. Folia Parasitol (Praha) 49:319–322
Ehigiator HN, Romagnoli P, Borgelt K et al (2005) Mucosal cytokine and antigen specific responses to Cryptosporidium parvum in IL-12p40 KO mice. Parasite Immunol 27:17–28
Ehigiator HN, Romagnoli P, Priest JW, Secor WE, Mead JR (2007) Induction of murine immune responses by DNA encoding a 23-kDa antigen of Cryptosporidium parvum. Parasitol Res 101:943–950
Galen JE, Pasetti MF, Tennant S, Ruiz-Olvera P, Sztein MB, Levine MM (2009) Salmonella enterica serovar Typhi live vector vaccines finally come of age. Immunol Cell Biol 5:1–13
Grangette C, Muller-Alouf H, Goudercourt D et al (2001) Mucosal immune responses and protection against tetanus toxin after intranasal immunisation with recombinant Lactobacillus plantarum. Infect Immunol 69:1547–1553
Griffiths JK (1998) Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment, and diagnosis. Adv Parasitol 40:37–85
Jakobi V, Petry F (2006) Differential expression of Cryptosporidium parvum genes encoding sporozoite surface antigens in infected HCT-8 host cells. Microbes Infect 8:2186–2194
Leav BA, Mackay MR, Anyanwu A et al (2002) Analysis of sequence diversity at the highly polymorphic Cpgp40/15 locus among Cryptosporidium isolates from human immunodeficiency virus-infected children in South Africa. Infec Immun 70:3881–3890
Li YJ, Ma GP, Li GW, et al. (2010) Oral vaccination with the porcine rotavirus VP4 outer capsid protein expressed by Lactococcus lactis induces specific antibody production. J Biomed Biotechnol. doi:10.1155/2010/708460
Lucca P, De Gaspari EN, Bozzoli LM et al (2009) Molecular characterization of Cryptosporidium spp. from HIV infected patients from an urban area of Brazil. Rev Inst Med Trop Sao Paulo 51:341–343
Makino SI, Watarai M, Tabuchi H et al (2001) Genetically modified shiga toxin 2e (Stx2e) producing Escherichia coli is a vaccine candidate for porcine edema disease. Microb Pathog 31:1–8
Mead JR, You X (1998) Susceptibility differences to Cryptosporidium parvum infection in two strains of gamma interferon knockout mice. J Parasitol 84:1045–1048
Mead JR (2010) Challenges and prospects for a Cryptosporidium vaccine. Future Microbiol 5:335–337
Medina E, Guzmán CA (2001) Use of live bacterial vaccine vectors for antigen delivery: potential and limitations. Vaccine 19:1573–1580
Mondal D, Haque R, Sack RB, Kirkpatrick BD, Petri WA Jr (2009) Attribution of malnutrition to cause-specific diarrheal illness: evidence from a prospective study of preschool children in Mirpur, Dhaka, Bangladesh. Am J Trop Med Hyg 80:824–826
Mor SM, Tzipori S (2008) Cryptosporidiosis in children in Sub-Saharan Africa: a lingering challenge. Clin Infect Dis 47:915–921
Okhuysen PC, Chappell CL, Sterling CR, Jakubowski W, DuPont HL (1998) Susceptibility and serologic response of healthy adults to reinfection with Cryptosporidium parvum. Infect Immun 66:441–443
Perryman LE, Kapil SJ, Jones ML, Hunt EL (1999) Protection of calves against cryptosporidiosis with immune bovine colostrum induced by a Cryptosporidium parvum recombinant protein. Vaccine 23:2142–2149
Ramasamy R, Yasawardena Y, Zomer A, Venema G, Kok J, Leenhouts K (2006) Immunogenicty of a malaria parasite antigen displayed by Lactococcus lactis in oral immunizations. Vaccine 24:3900–3908
Ribeiro LA, Azevedo V, Le Loir Y et al (2002) Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: a first step towards food-grade live vaccines against Brucellosis. Appl Environ Microbiol 12:910–916
Riggs MW (2002) Recent advances in cryptosporidiosis: the immune response. Microbes Infect 4:1067–1080
Scheppler L, Vogel M, Zuercher AW et al (2002) Recombinant Lactobacillus johnsonii as a mucosal vaccine delivery vehicle. Vaccine 20:2913–2920
Seegers JF (2002) Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends Biotechnol 20:508–515
Shirafuji H, Xuan X, Kimata I et al (2005) Expression of P23 of Cryptosporidium parvum in Toxoplasma gondii and evaluation of its protective effects. J Parasitol 91:476–479
Singh I, Theodos C, Li W, Tzipori S (2005) Kinetics of Cryptosporidium parvum-specific cytokine responses in healing and nonhealing murine models of C. parvum infection. Parasitol Res 97:309–317
Steidler L, Hans W, Schotte L et al (2000) Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352–1355
Takashima Y, Xuan X, Kimata I et al (2003) Recombinant bovine herpesvirus-1 expressing p23 protein of Cryptosporidium parvum induces neutralizing antibodies in rabbits. J Parasitol 89:276–282
Tessema TS, Dauber E, Petry F (2009) Adoptive transfer of protective immunity from Cryptosporidium parvum-infected interferon-gamma and interleukin-12-deficient mice to naive recipients. Vaccine 27:6575–6581
van de Guchte M, van der Vossen JM, Kok J, Venema G (1989) Construction of a lactococcal expression vector: expression of hen egg white lysozyme in Lactococcus lactis subsp. lactis. Appl Environ Microbiol 55:224–228
von Oettingen J, Nath-Chowdhury M, Ward BJ, Rodloff AC, Arrowood MJ, Ndao M (2008) High-yield amplification of Cryptosporidium parvum in interferon gamma receptor knockout mice. Parasitology 135:1151–1156
Wyatt CR, Perryman LE (2003) Detection of mucosally delivered antibody to Cryptosporidium parvum p23 in infected calves. J Parasitol 89:918–923
Wang HF, Swain JB, Besser TE, Jasmer D, Wyatt CR (2000) Detection of antibodies to a recombinant Cryptosporidium parvum p23 in serum and feces from neonatal calves. Ann N Y Acad Sci 916:378–387
Xin KQ, Hoshino Y, Toda Y et al (2003) Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood 102:223–228
Ya T, Zhang Q, Chu F et al (2008) Immunological evaluation of Lactobacillus casei Zhang: a newly isolated strain from koumiss in Inner Mongolia, China. BMC Immunol 9:68
Yao XY, Yuan MM, Li DJ (2007) Molecular adjuvant C3d3 improved the anti-hCG beta humoral immune response in vaginal inoculation with live recombinant Lactobacillus expressing hCG beta-C3d3 fusion protein. Vaccine 25:6129–6139
Acknowledgments
This work was supported by China Natural Science Foundation (Grant No. 30960279) and the Postdoctoral Foundation of Inner Mongolia Agricultural University (No. 57545).
Author information
Authors and Affiliations
Corresponding author
Additional information
Geriletu and Rihua Xu equally contributed to this work.
Rights and permissions
About this article
Cite this article
Geriletu, Xu, R., Jia, H. et al. Immunogenicity of Orally Administrated Recombinant Lactobacillus casei Zhang Expressing Cryptosporidium parvum Surface Adhesion Protein P23 in Mice. Curr Microbiol 62, 1573–1580 (2011). https://doi.org/10.1007/s00284-011-9894-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-011-9894-4