Abstract
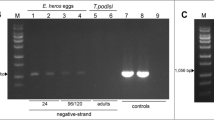
We have used real-time quantitative PCR to measure, for the first time, the relative phage WO-B orf7 density and infection incidence in Aedes albopictus mosquitoes from fields in Thailand. Our results showed that the infection incidence of phage WO-B in this mosquito, sampled from geographically different places in Thailand, was 97.9%. Average relative densities of the offspring were different when collected from diverse parts and reared under the same conditions in the laboratory. Our results also revealed that geographical differences within Thailand did not influence the maternal transmission rate of bacteriophage WO-B. In addition, the orf7 loci might not be strictly associated with Wolbachia, because less than 100% of them were maternally inherited. This discovery does not support the hypothesis that bacteriophage WO-B is involved in Aedes albopictus’ cytoplasmic incompatibility. Whether this bacteriophage actually is involved in Wolbachia-induced cytoplasmic incompatibility in this mosquito thus needs further investigation, and additional densities of phage WO-B loci should be integrated.

Similar content being viewed by others
References
Ahantarig A, Khumthong R, Kittayapong P, Baimai V (2008) Relative densities of bacteriophage WO and Wolbachia of Aedes albopictus mosquito during development. Ann Microbiol 58:189–193
Bordenstein S, Marshall ML, Fry AJ, Kim U, Wernegreen JJ (2006) The tripartite associations between bacteriophage, Wolbachia, and arthropods. PLoS Pathogens 2:e43
Buei K (1983) Pictorial key to species. Adult mosquitoes in Thailand. Ministry of Public Health, Bangkok
Chauvatcharin N, Ahantarig A, Baimai V, Kittayapong P (2006) Bacteriophage WO-B and Wolbachia in natural mosquito hosts: infection incidence, transmission mode and relative density. Mol Ecol 15:2451–2461
Duron O, Fort P, Weill M (2006) Hypervariable prophage WO sequences describe an unexpected high number of Wolbachia variants in the mosquito Culex pipiens. Proc Biol Sci 273:495–502
Fujii Y, Kubo T, Ishikawa H, Sasaki T (2004) Isolation and characterization of the bacteriophage WO from Wolbachia, an arthropod endosymbiont. Biochem Biophys Res Commun 317:1183–1188
Gavotte L, Henri H, Stouthamer R, Charif D, Charlat S, Bouletreau M, Vavre F (2007) A survey of the bacteriophage WO in the endosymbiotic bacteria Wolbachia. Mol Biol Evol 24:427–435
Guillemaud T, Pasteur N, Rousset F (1997) Contrasting levels of variability between cytoplasmic genomes and incompatibility types in the mosquito Culex pipiens. Proc Biol Sci 264:245–251
Iturbe-Ormaetxe I, Burke GR, Riegler M, O’Neill SL (2005) Distribution, expression, and motif variability of ankyrin domain genes in Wolbachia pipientis. J Bacteriol 187:5136–5145
Kambhampati S, Rai KS (1991) Mitochondrial DNA variation within and among populations of the mosquito, Aedes albopictus. Genome 34:288–292
Kambhampati S, Black WC, Rai KS (1991) Geographic origin of the US and Brazilian Aedes albopictus inferred from allozyme analysis. Heredity 67:85–94
Knudsen AB (1995) Global distribution and continuing spread of Aedes albopictus. Parasitologia 37:91–97
Masui S, Kamoda S, Sasaki T, Ishikawa H (2000) Distribution and evolution of bacteriophage WO in Wolbachia, the endosymbiont causing sexual alterations in arthropods. J Mol Evol 51:491–497
O’Neill SL, Giordane R, Colbert AME, Karr TL, Robertsu HM (1992) 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with CI in insects. Proc Natl Acad Sci USA 89:2699–2702
Rattanarithikul R, Panthusiri P (1994) Illustrated keys to the medically important mosquitoes of Thailand. Wattana Panich Press, Bangkok
Ruang-areerate T, Kittayapong P (2006) Wolbachia transfection in Aedes aegypti: a potential gene driver of dengue vectors. Proc Natl Acad Sci USA 103:12534–12539
Sanogo YO, Dobson SL (2004) Molecular discrimination of Wolbachia in the Culex pipiens complex: evidence for variable bacteriophage hyperparasitism. Insect Mol Biol 13:365–369
Schilthuizen M, Stouthamer R (1997) Horizontal transmission of parthenogenesis-inducing microbes in Trichogramma wasps. Proc R Soc Lond B Sci 264:361–366
Sinkins SP, Walker T, Lynd AR, Steven AR, Makepeace BL, Godfray HC, Parkhill J (2005) Wolbachia variability and host effects on crossing type in Culex mosquitoes. Nature 436:257–260
Stouthamer R, Breeuwer JA, Hurst GD (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53:71–102
Werren JH, Windsor DM (2000) Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc R Soc Lond B 267:1277–1285
Wu M, Sun LV, Vamathevan J, Riegier M, Deboy R et al (2004) Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol 2:e69. doi:10.1371/journal.pbio.020069
Acknowledgments
We are grateful for anonymous reviewer(s) for the advice to improve this article during the review process. We also thank Dr. John R. Milne for reviewing the manuscript; Drs. Ronald Morales Vargas, Supanee Hirunkanokpun, and Supat Wiwatanaratanabutr for their helpful suggestions; and Mr. Kitti Theinthong, Ms. Samnieng Theinthong, and Miss Nutchaya Klinpikul for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahantarig, A., Chauvatcharin, N., Ruang-areerate, T. et al. Infection Incidence and Relative Density of the Bacteriophage WO-B in Aedes albopictus Mosquitoes from Fields in Thailand. Curr Microbiol 62, 816–820 (2011). https://doi.org/10.1007/s00284-010-9769-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9769-0