Abstract
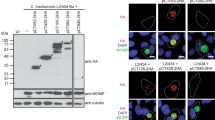
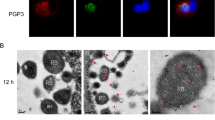
To localize and characterize the GTP-binding protein encoded by the chlamydial ORF CT703 in the Chlamydia trachomatis-infected cells, the gene coding for CT703 in the Chlamydia trachomatis serovar L2 genome was cloned into the prokaryotic expression vector pGEX and expressed as GST fusion protein in the E. coli BL21 strain. The GST-CT703 fusion protein was purified and used to raise antigen-specific antibodies. Using the anti-fusion protein antibodies, we localized the endogenous CT703 protein inside the chlamydial inclusion using an indirect immunofluorescence assay (IFA). We also detected a significantly decreased level of CT703 in cultures that were induced to undergo persistent infection. These observations suggest that CT703 may be an important regulator for promoting chlamydial productive infection.







Similar content being viewed by others
References
Askienazy-Elbharl M, Henry-Suchet J (1999) Persistent “Silent” Chlamydia trachomatis female genital tract infections. Infections Dis Obstet Gynecol 7:31–34
Beatty WL, Byrne GI, Morrison RP (1993) Morphologic and antigenic characterization of interferon γ-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA 90:3998–4002
Beatty WL, Morrison RP et al (1994) Immunoelectron-microscopic quantitation of differential levels of chlamydial proteins in a cells culture model of persistent Chlamydia trachomatis infection. Infect Immun 62:4059–4062
Belland RJ, David E, Virok D et al (2003) Transcription analysis of chlamydial growth IFN-γ-mediated persistence and reactivation. PNAS 100:15971–15976
Carabeo RA, Mead DJ, Hackstadt T (2003) Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. PNAS 100:6771–6776
Chen CQ, Chen D, Sharma J et al (2006) The hypothetical protein CT813 is localized in the Chlamydia trachomatis inclusion membrane and is immunogenic in women urogenitally infected with C. trachomatis. Infect Immun 74:4826–4840
Fan T, Lu H, Hu H et al (1998) Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496
Hackstadt T (1998) The diverse habitats of obligate intracellular parasites. Curr Opin Microbiol 1:82–87
Hackstadt T, Scidmore MA, DRockey D (1995) Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. PNAS 92:4877–4881
Hackstadt T, Rockey DD, Heinzen RA et al (1996) Chiamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J 15:964–977
Hackstadt T, Fischer ER, Scidmore MA et al (1997) Origins and functions of the chlamydial inclusion. Trends Microbiol 5:288–293
Hybiske K, Stephens RS (2007) Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc Natl Acad Sci USA 104:11430–11435
Kuipers JG, Zeidler H, Kohler L (2003) How does Chlamydia cause arthritis? Rheum Dis Clin North Am 29:613–629
Pinkerton SD, Layde PM, For the NIMH multisite HIV prevention trial group (2002) Using sexually transmitted disease incidence as a surrogate marker for HIV incidence in prevention trials: a modeling study. Sex Transm Dis 29:298–307
Polkinghorn A, Hogan RJ, Vaughan L et al (2006) Differential expression of chlamydial signal transduction genes in normal and interferon gamma-induced persistent Chlamydophila pneumoniae infections. Microbes Infect 8:61–72
Rzomp KA, Scholtes LD, Briggs BJ et al (2003) Rab GTPases are recruited to Chlamydial inclusions in both a species-dependent and species-independent manner. Infect Immun 71:5855–5870
Sambrook J, Ruessell DW (2002) Molecular cloning: a laboratory manual, 3rd edn. Science Press, Beijing, pp 1217–1265
Scidmore MA, Fischer ER, Hackstadt T (1996) Sphingolipids and glycoproteins are differentially trafficked to the Chlamydia trachomatis inclusion. J Cell Biol 134:363–374
Scidmore MA, Fischer ER, Hackstadt T (2003) Restricted fusion of Chlamydia trachomatis vesicles with endocytic compartments during the initial stages of infection. Infect Immun 71:973–984
Sherman KJ, Daling JR, Stergachis A et al (1990) Sexually transmitted diseases and tubal pregnancy. Sex Transm Dis 17:115–121
Stephens RS, Kalman S, Lammel C et al (1998) Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754–759
Su H, McClarty G, Dong F et al (2004) Activation of Raf/MEK/ERK/cPLA2 signaling pathway is essential for Chlamydia acquisition of host glycerophomatis. J Biol Chem 279:9409–9416
Taylor HR, Johnson SL, Schachter J et al (1987) Pathogenesis of trachoma: the stimulus for inflammation. J Immunol 138:3023–3027
Wallin KL, Wiklund F, Luostarinen T et al (2002) A population-based prospective study of Chlamydia trachomatis infection and cervical carcinoma. Int J Cancer 101:371–374
Xiao Y, Zhong Y, Greene W et al (2004) Chlamydia trachomatis infection inhibits both Bax and Bak activation induced by staurosporine. Infect Immun 72:5470–5474
Zhong G, Fan P, Ji H et al (2001) Identification of a Chlamydial protease-like activity factor responsible for the degradation of host transcription factor. J Exp Med 193:935–942
Acknowledgment
Supported by the National Natural Science Foundation of China (Grand No. 30600534).
Author information
Authors and Affiliations
Corresponding author
Additional information
Kun Du and FuYan Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Du, K., Wang, F., Huo, Z. et al. Localization and Characterization of GTP-Binding Protein CT703 in the Chlamydia trachomatis-Infected Cells. Curr Microbiol 62, 465–471 (2011). https://doi.org/10.1007/s00284-010-9730-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9730-2