Abstract
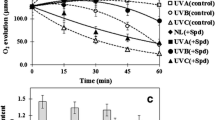
To investigate the short term effect of ultraviolet (UV) radiations on changes in pigments and polyamine contents, Synechocystis sp. PCC 6803 cells after exposure to UV-radiation were extracted by dimethylformamide and perchloric acid for pigments and polyamines determination, respectively. Cell growth was slightly decreased after 1 h exposure to UV-A and UV-B radiations. UV-C had little effect on cell growth despite the decrease of photosynthetic rate by about 18%. UV-A and UV-B decreased the contents of chlorophyll a and carotenoids whereas UV-C decreased chlorophyll a but had no effect on carotenoids. Spermidine contents were unaffected by UV-A, in contrast to the reduction of 25 and 50% by UV-B and UV-C, respectively. All three types of UV-radiation particularly reduced perchloric acid-insoluble spermidine. Importantly, putrescine and spermine which accounted for less than 1% of intracellular polyamines were increased by about three- to eight-fold by UV-B and UV-C, respectively. The changes in polyamines contents by UV-B and UV-C were consistent with the changes in transcript levels of arginine decarboxylase mRNA, but not with the protein levels. The decrease in the transcripts of adc2 but not adc1 was observed with UV-B and UV-C treatments.



Similar content being viewed by others
Abbreviations
- ADC:
-
Arginine decarboxylase
- PCA:
-
Perchloric acid
- Put:
-
Putrescine
- Spd:
-
Spermidine
- Spm:
-
Spermine
- UV:
-
Ultraviolet
References
Bank HL, John J, Schmehl MK et al (1990) Bactericidal effectiveness of modulated UV light. Appl Environ Microbiol 56:3888–3889
Barbato R, Bergo E, Szabò I et al. (2000) Ultraviolet B exposure of whole leaves of barley affects structure and functional organization of photosystem II. J Biol Chem 275:10976–10982
Bavcon J, Gaberščik A, Batič F (1996) Influence of UV-B radiation on photosynthetic activity and chlorophyll fluorescence kinetics in Norway spruce [Picea abies (L.) Karst.] seedlings. Trees 10:172–176
Borrell A, Culiañez-Macià FA, Altabella T et al (1995) Arginine decarboxylase is localized in chloroplasts. Plant Physiol 109:771–776
Bouchereau A, Aziz A, Larher F et al (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Brandle JR, Campbell WF, Sisson WB et al (1977) Net photosynthesis, electron transport capacity, and ultrastructure of Pisum sativum L. exposed to ultraviolet-B radiation. Plant Physiol 60:165–169
Brandt AM, Raksajit W, Yodsang P et al. (2010) Characterization of the substrate-binding PotD subunit in Synechocystis sp. strain PCC 6803. Arch Microbiol (in press)
Caldwell MM, Teramura AH, Tevini M (1989) The changing solar ultraviolet climate and the ecological consequences for higher plants. Trends Ecol Evol 4:363–367
Chamovitz D, Sandmann G, Hirschberg J (1993) Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J Biol Chem 268:17348–17353
Cullen JJ, Neale PJ, Lesser MP (1992) Biological weighting function for the inhibition of phytoplankton photosynthesis by ultraviolet radiation. Science 258:646–650
Evans PT, Malmberg RL (1989) Do polyamines have roles in plant development? Annu Rev Plant Physiol Plant Mol Biol 40:235–529
Galston AW, Kaur-Sawhney R (1995) Polyamines as endogenous growth regulators. In: Davies PJ (ed) Plant hormones: physiology, biochemistry, and molecular biology. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 158–178
Garcia-Jimenez P, Rodrigo M, Robaina RR (1998) Influence of plant growth regulators, polyamines and glycerol interaction on growth and morphogenesis of carposporelings of Grateloupia cultured in vitro. J Appl Phycol 10:95–100
Groppa MD, Benavides MP (2008) Polyamines and abiotic stress: recent advances. Amino Acids 34:35–45
Ha HC, Sirisoma NS, Kuppusamy P et al (1998) The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci USA 95:11140–11145
He Y-Y, Häder D-P (2002) Reactive oxygen species and UV-B: effect on cyanobacteria. Photochem Photobiol Sci 1:729–736
Incharoensakdi A, Jantaro S, Raksajit W et al (2010) Polyamines in cyanobacteria: biosynthesis, transport and abiotic stress response. In: Méndez-Vilas A (ed) Current research, technology and education topics in applied microbiology and microbial biotechnology. Microbiology book series, Formatex, Spain (in press)
Ioannidis NE, Ortigosa SM, Veramendi J et al (2009) Remodeling of tobacco thylakoids by over-expression of maize plastidial transglutaminase. Biochim Biophys Acta 1787:1215–1222
Jantaro S, Incharoensakdi A, Jansén T et al (2005) Effects of long-term ionic and osmotic stress conditions on photosynthesis in the cyanobacterium Synechocystis sp. PCC 6803. Funct Plant Biol 32:807–815
Jantaro S, Mäenpää P, Mulo P et al (2003) Content and biosynthesis of polyamines in salt and osmotically stressed cells of Synechocystis sp. PCC 6803. FEMS Microbiol Lett 228:129–135
Jürgens U, Golecki JR, Weckesser J (1985) Characterization of the cell wall of the unicellular cyanobacterium Synechocystis PCC 6714. Arch Microbiol 142:168–174
Kim D-S, Watanabe Y (1993) The effect of long wave ultraviolet radiation (UV-A) on the photosynthetic activity of natural population of freshwater phytoplankton. Ecol Res 8:225–234
Lütz C, Navakoudis E, Seidlitz HK et al (2005) Simulated solar irradiation with enhanced UV-B adjust plastid- and thylakoid-associated polyamine changes for UV-B protection. Biochim Biophys Acta 1710:24–33
McFarland M, Kaye J (1992) Chlorofluorocarbons and ozone. Photochem Photobiol 55:911–929
Mohamed A, Jansson C (1989) Influence of light in accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol Biol 13:693–700
Moran R (1982) Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol 69:1376–1381
Rahmatzadeh S, Khara J (2007) Anatomical and morphological changes caused by interaction between UV-C radiation and colonized wheat by some species of arbuscular mycorrhizas. J Biol Sci 7:1001–1004
Regunathan S, Reis DJ (2000) Characterization of arginine decarboxylase in rat brain and liver: distinction from ornithine decarboxylase. J Neurochem 74:2201–2208
Raksajit W, Mäenpää P, Incharoensakdi A (2006) Putrescine transport in a cyanobacterium Synechocystis sp. PCC 6803. J Biochem Mol Biol 39:394–399
Redmond JW, Tseng A (1979) High-pressure liquid chromatographic determination of putrescine, cadaverine, spermidine and spermine. J Chromatogr 170:479–481
Ries G, Buchholz G, Frohnmeyer H et al (2000) UV-damage mediated induction of homologous recombination in Arabidopsis is dependent on photosynthetically active radiation. Proc Natl Acad Sci USA 97:13425–13429
Rinalducci S, Hideg É, Vass I et al (2006) Effect of moderate UV-B irradiation on Synechocystis PCC 6803 biliproteins. Biochem Biophys Res Commun 341:1105–1112
Santos I, Fidalgo F, Almeida JM et al (2004) Biochemical and ultrastructural changes in leaves of potato plants grown under supplementary UV-B radiation. Plant Sci 167:925–935
Sfichi L, Ioannidis N, Kotzabasis K (2004) Thylakoid-associated polyamines adjust the UV-B sensitivity of the photosynthetic apparatus by means of light-harvesting complex II changes. Photochem Photobiol 80:499–506
Sfichi-Duke L, Ioannidis NE, Kotzabasis K (2008) Fast and reversible response of thylakoid-associated polyamines during and after UV-B stress: a comparative study of the wild type and a mutant lacking chlorophyll b of unicellular green alga Scenedesmus obliquus. Planta 228:341–353
Shimizu N, Hosogi N, Hyon G-S et al (2006) Reactive oxygen species (ROS) generation and ROS-induced lipid peroxidation are associated with plasma membrane modifications in host cells in response to AK-toxin I from Alternaria alternata Japanese pear pathotype. J Gen Plant Pathol 72:6–15
Simpson WR, von Glasow R, Riedel K et al (2007) Halogens and their role in polar boundary-layer ozone depletion. Atmos Chem Phys 7:4375–4418
Smith JL, Burritt DJ, Bannister P (2000) Shoot dry weight, chlorophyll and UV-B-absorbing compounds as indicators of a plant’s sensitivity to UV-B radiation. Ann Bot 86:1057–1063
Smith JL, Burritt DJ, Bannister P (2001) Ultraviolet-B radiation leads to a reduction in free polyamines in Phaseolus vulgaris L. Plant Growth Regul 35:289–294
Sommaruga R, Libkind D, van Broock M et al (2006) Mycosporine-glutaminol-glucoside, a UV-absorbing compound of two Rhodotorula yeast species. Yeast 21:1077–1081
Stapleton AE (1992) Ultraviolet radiation and plants: burning questions. Plant Cell 4:1353–1358
Urano K, Yoshiba Y, Nanjo T et al (2004) Arabidopsis stress-inducible gene for arginine decarboxylase AtADC2 is required for accumulation of putrescine in salt tolerance. Biochem Biophys Res Commun 313:369–375
Voigt J, Deinert B, Bohley P (2000) Subcellular localization and light-dark control of ornithine decarboxylase in the unicellular alga Chlamydomonas reinhardtii. Physiol Plant 108:353–360
Yamaguchi K, Takahashi Y, Berberich T et al (2006) The polyamine spermine protects against high salt stress in Arabidopsis thaliana. FEBS Lett 580:6783–6788
Yoda H, Yamaguchi Y, Sano H (2003) Induction of hypersensitive cell death by hydrogen peroxide produced through polyamine degradation in tobacco plants. Plant Physiol 132:1973–1981
Zacchini M, de Agazio M (2004) Spread of oxidative damage and antioxidative response through cell layers of tobacco callus after UV-C treatment. Plant Physiol Biochem 42:445–450
Zhao F, Song C-P, He J et al (2007) Polyamines improve K+/Na+ homeostasis in barley seedlings by regulating root ion channel activities. Plant Physiol 145:1061–1072
Acknowledgments
The ADC antibody was provided by Dr. Nanthika Kongcharoenporn, The Institute of Biotechnology and Genetic Engineering, Chulalongkorn University. This work was supported by TRF-CHE Research Grant for New Scholar, the Thailand Research Fund (TRF) in conjunction with the Commission on Higher Education (CHE), Ministry of Education, Thailand, and start-up fund provided by Chulalongkorn University to S.J. The support from CHE (university staff development consortium) and from the Thai Government Stimulus Package 2 (TKK 2555) under the Project for Establishment of Comprehensive Center for Innovative Food, Health Products and Agriculture (PER FECTA) to A.I. are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jantaro, S., Pothipongsa, A., Khanthasuwan, S. et al. Short-Term UV-B and UV-C Radiations Preferentially Decrease Spermidine Contents and Arginine Decarboxylase Transcript Levels of Synechocystis sp. PCC 6803. Curr Microbiol 62, 420–426 (2011). https://doi.org/10.1007/s00284-010-9724-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9724-0