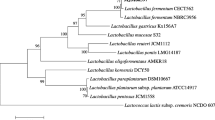
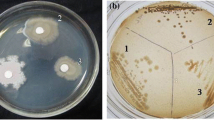
Abstract
In this study, Lactobacillus fermentum (L. fermentum) F1 reduced cholesterol 48.87%. The strain was screened from cattle feces using an API 50 CHL system and the 16S rRNA sequence contrasting method. L. fermentum F1 showed acid and bile tolerance, and antimicrobial activity against pathogenic Escherichia coli and Staphylococcus aureus. L. fermentum F1 deconjugated 0.186 mM of free cholalic acid after it was incubated at 37°C in 0.20% sodium taurocholate (TCA) broth for 24 h. Heat-killed and resting cells of L. fermentum F1 showed small amounts of cholesterol removal (6.85 and 25.19 mg/g, respectively, of dry weight) compared with growing cells (33.21 mg/g of dry weight). The supernatant fluid of the broth contained 50.85% of the total cholesterol, while the washing buffer and cell extracts had 13.53 and 35.39%, respectively. These findings suggest that L. fermentum F1 may remove cholesterol by co-precipitating with deconjugated bile salt, assimilating with cells and by incorporation into cellular membranes. Cholesterol assimilated by cells held 72.0% of the reduced cholesterol, while 21.65% of the reduced cholesterol was coprecipitated with deconjugated bile salt and 5.89% was adsorbed into the surface of the cells.




Similar content being viewed by others
References
Ranade VV (1993) Significance of cholesterol in health and disease. Int J Clin Pharmacol Ther Toxicol 31:276–284
Tabas I (2002) Cholesterol in health and disease. J Clin Invest 110:583–590
Harrison VC, Peat G (1975) Serum cholesterol and bowel flora in the newborn. Am J Clin Nutr 28:1351–1355
Endo T, Nakano M, Shimizu S, Fukushima M, Miyoshi S (1999) Effects of a probiotic on the lipid metabolism of cocks fed on a cholesterol-enriched diet. Biosci Biotechnol Biochem 63:1569–1575
Sanders TA (1999) Food production and food safety. Bmj 318:1689–1693
Gilliland SE, Nelson CR, Maxwell C (1985) Assimilation of cholesterol by Lactobacillus acidophilus. Appl Environ Microbiol 49:377–381
Gilliland SE, Walker DK (1990) Factors to consider when selecting a culture of Lactobacillus acidophilus as a dietary adjunct to produce a hypocholesterolemic effect in humans. J Dairy Sci 73:905–911
Klaver FA, van der Meer R (1993) The assumed assimilation of cholesterol by Lactobacilli and Bifidobacterium bifidum is due to their bile salt-deconjugating activity. Appl Environ Microbiol 59:1120–1124
Noh DO, Kim SH, Gilliland SE (1997) Incorporation of cholesterol into the cellular membrane of Lactobacillus acidophilus ATCC 43121. J Dairy Sci 80:3107–3113
Rudel LL, Morris MD (1973) Determination of cholesterol using o-phthalaldehyde. J Lipid Res 14:364–366
Pan D, Zhang D (2005) Screening of cholesterol reducing lactic acid bacteria and its activity in cholesterol reducing. Food Sci 26:233–237
Usman HA (1999) Bile tolerance, taurocholate deconjugation, and binding of cholesterol by Lactobacillus gasseri strains. J Dairy Sci 82:243–248
Irvin JL, Johnson CG, Kopalo J (1944) A photometric method of the determination of cholates in bile and blood. J Biol Chem 153:439
Liong MT, Shah NP (2005) Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. J Dairy Sci 88:55–66
Razin S, Kutner S, Efrati H, Rottem S (1980) Phospholipid and cholesterol uptake by mycoplasma cells and membranes. Biochim Biophys Acta 598:628
Usman HA (1999) Viability of Lactobacillus gasseri and its cholesterol-binding and antimutagenic activities during subsequent refrigerated storage in nonfermented milk. J Dairy Sci 82:2536–2542
Mishra V, Prasad DN (2005) Application of in vitro methods for selection of Lactobacillus casei strains as potential probiotics. Int J Food Microbiol 103:109–115
Temmerman R, Pot B, Huys G, Swings J (2003) Dentification and antibiotic susceptibility of bacterial isolates from probiotic products. Int J Food Microbiol 81:1–10
Vanderhoof JA, Whitneyx DB, Antonson DL (1999) Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr 35:564–568
Acknowledgments
This work was supported by the State Science and Technology Ministry of the People’s Republic of China (programs no. 2007AA10Z357, 2009C22014, and 2006BAD27B09), the Natural Science Ministry of China (program no. 30972130) and the K. C. Wong Magna Fund, Ningbo University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, X.Q., Pan, D.D. & Zhou, P.D. Functional Characteristics of Lactobacillus fermentum F1. Curr Microbiol 62, 27–31 (2011). https://doi.org/10.1007/s00284-010-9669-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9669-3